��Ŀ����
����Ŀ��������̼����������ЧӦ������Ҫ���ʣ�������Ч�Ļ������ã������ܻ�����ԴΣ�����ֿɼ�������ЧӦ��Ӱ�죬���н����Դ����ͻ��������˫�����塣
(1)![]() ��
��![]() �������������Եõ��ϳ�����
�������������Եõ��ϳ�����![]() ��
��![]() ����
����
![]()
��һ���¶Ⱥ�ѹǿ�£���Ԫ�����ȶ��ĵ�������![]() ��������ʱ�ķ�Ӧ�ȳ�Ϊ�û�����ı�Ħ�������ʡ���֪
��������ʱ�ķ�Ӧ�ȳ�Ϊ�û�����ı�Ħ�������ʡ���֪![]() ��
��![]() ��
��![]() �ı�Ħ�������ʷֱ�Ϊ
�ı�Ħ�������ʷֱ�Ϊ![]() ��
��![]() ��
��![]() ��������������Ӧ��
��������������Ӧ��![]() ________
________![]() ��
��
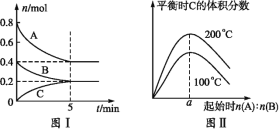
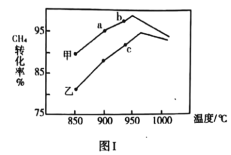
������������ͬ���ס������ֲ�ͬ���������£���ͬʱ���ڲ��![]() ת����
ת����![]() ���¶ȱ仯��ϵ��ͼ��
���¶ȱ仯��ϵ��ͼ��![]() ________�������һ������һ��δ�����ﵽƽ��״̬������________��
________�������һ������һ��δ�����ﵽƽ��״̬������________��
(2)![]() ��
��![]() ����������������
����������������![]() ���䷴Ӧ����Ϊ��
���䷴Ӧ����Ϊ��![]() ��
��
��![]() ʱ����
ʱ����![]() ���ܱ������г���
���ܱ������г���![]() ��
��![]() ģ�ҵ������Ͷ�ϱ�
ģ�ҵ������Ͷ�ϱ�![]() ����ͼ����
����ͼ����![]() ƽ��ת����
ƽ��ת����![]() ��
��![]() �Ĺ�ϵ����ͼ��
�Ĺ�ϵ����ͼ��![]() ��
��![]() ��ƽ��ת����
��ƽ��ת����![]() _______��
_______��
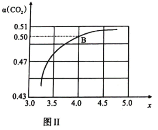
�ڵ�![]() ʱ������ʼ��ѹǿΪ
ʱ������ʼ��ѹǿΪ![]() ��ˮΪҺ̬��ƽ��ʱѹǿ��Ϊ��ʼ��
��ˮΪҺ̬��ƽ��ʱѹǿ��Ϊ��ʼ��![]() ����ƽ���ѹ����ѹ=��ѹ�����ʵ�������������ƽ��Ũ�ȱ�ʾ�÷�Ӧ��ƽ�ⳣ��
����ƽ���ѹ����ѹ=��ѹ�����ʵ�������������ƽ��Ũ�ȱ�ʾ�÷�Ӧ��ƽ�ⳣ��![]() ________
________![]() ��
��
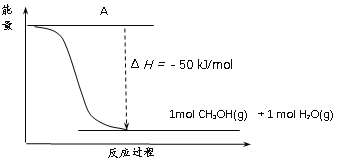
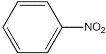
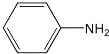
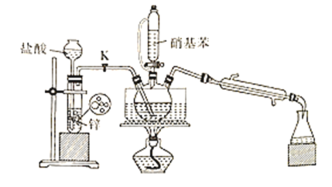
(3)�Զ������ѱ��渲��![]() Ϊ���������Խ�
Ϊ���������Խ�![]() ��
��![]() ֱ��ת�������ᡣ
ֱ��ת�������ᡣ
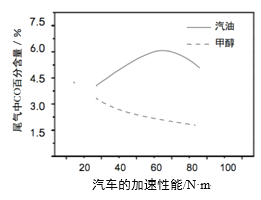
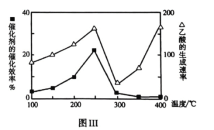
���ڲ�ͬ�¶��´����Ĵ�Ч����������������ʵı仯�����ͼ����ʾ��250-300��ʱ���¶����߶�������������ʽ��͵�ԭ����________��
��Ϊ����߸÷�Ӧ��![]() ��ת���ʣ����Բ�ȡ�Ĵ�ʩ��________��
��ת���ʣ����Բ�ȡ�Ĵ�ʩ��________��
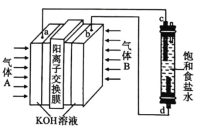
(4)����������Ĥ�е�![]() �������
�������![]() �����仹ԭ��������������ĤΪ�����缫����һ��Ũ�ȵ�����Ϊ���ʣ���һ��������ͨ��
�����仹ԭ��������������ĤΪ�����缫����һ��Ũ�ȵ�����Ϊ���ʣ���һ��������ͨ��![]() ���е�⣬���������Ƶõ��ܶȾ���ϩ
���е�⣬���������Ƶõ��ܶȾ���ϩ![]() �������
�������![]() �������ʱ��
�������ʱ��![]() ����ת��Ϊ
����ת��Ϊ![]() �ĵ缫��Ӧʽ��________��
�ĵ缫��Ӧʽ��________��
���𰸡�![]() һ��δ ��Ϊ��ͬ�¶�ʱ
һ��δ ��Ϊ��ͬ�¶�ʱ![]() �������ƽ�⣬��ͬ�¶�ʱ�������ת����Ӧ���
�������ƽ�⣬��ͬ�¶�ʱ�������ת����Ӧ��� ![]()
![]() �¶ȳ���
�¶ȳ���![]() ʱ�������Ĵ�Ч�ʽ��� ����ѹǿ�������Ӷ�����̼��Ũ��
ʱ�������Ĵ�Ч�ʽ��� ����ѹǿ�������Ӷ�����̼��Ũ�� ![]()
![]()
![]()
��������
(1)����Ӧ�ʱ�![]() ���ҵ��ʵı�Ħ��������Ϊ0������Ϣ���ٴ������ݼ��㼴�ɣ��ڴ�������ı�ƽ��ת���ʣ���ƽ��ʱ��������ͬ������ת����Ӧ����ͬ�ģ�
���ҵ��ʵı�Ħ��������Ϊ0������Ϣ���ٴ������ݼ��㼴�ɣ��ڴ�������ı�ƽ��ת���ʣ���ƽ��ʱ��������ͬ������ת����Ӧ����ͬ�ģ�
(2)ת����![]() ������������ʽ����Kpֵ��
������������ʽ����Kpֵ��
(3)��ͼ��ȡ��Ϣ�ɵ��¶����߶�������������ʽ��ͣ�˵���¶ȳ���250��ʱ�������Ĵ�Ч�ʽ��ͣ����û�ѧ����ʽ��![]() ���ж�����ʹ
���ж�����ʹ![]() ��ת�������͵�ʹƽ�������ƶ���������һ��Ӧ���������ϵѹǿ�����ԣ�
��ת�������͵�ʹƽ�������ƶ���������һ��Ӧ���������ϵѹǿ�����ԣ�
(4)�õ���غ�����д�缫��Ӧʽ��
(1)�ٸ��ݱ�Ħ�������ʵĶ��壬�ȶ����ʵı�Ħ��������Ϊ0����H2(g)��Ϊ0����Ӧ�ʱ�![]() ��
��
��c��һ��δ�ﵽƽ�⣻��Ϊ��������ı�ƽ��ת���ʣ�����ﵽƽ�⣬�״������Ҵ�������ͬ�¶ȵĵ��ཻ��ת����Ӧ��ȣ�
(2)��B��![]() ����
����![]() ��������̼��ת����Ϊ50%����Ӧ�Ķ�����̼Ϊ0.5mol������
��������̼��ת����Ϊ50%����Ӧ�Ķ�����̼Ϊ0.5mol������![]() ����Ӧ�İ���Ϊ1.0mol��������ת����
����Ӧ�İ���Ϊ1.0mol��������ת����![]() ��
��
�ڵ�x=1.0ʱ��![]() ������ʼѹǿΪ
������ʼѹǿΪ![]() ��ƽ��ʱѹǿΪ��ʼ��
��ƽ��ʱѹǿΪ��ʼ��![]() ����ƽ����ѹǿΪ
����ƽ����ѹǿΪ![]() ������ʼ�����Ͷ�����̼�����ʵ���Ϊ1mol�����ĵĶ�����̼���ʵ���Ϊx��
������ʼ�����Ͷ�����̼�����ʵ���Ϊ1mol�����ĵĶ�����̼���ʵ���Ϊx��
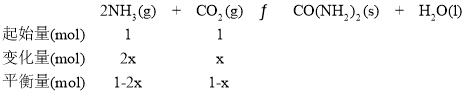
![]()
![]() ���������ʵ�������
���������ʵ�������![]() ����
����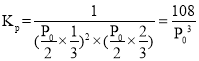 ��
��
(3)���¶ȳ���250��ʱ�������Ĵ�Ч�ʽ��ͣ������¶����߶�������������ʽ��ͣ�
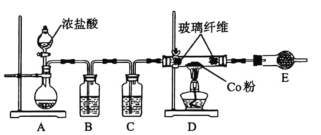
�ڽ�![]() ��
��![]() ֱ��ת��������Ļ�ѧ����ʽ��
ֱ��ת��������Ļ�ѧ����ʽ��![]() ��Ϊ�����
��Ϊ�����![]() ��ת���ʣ�������ѹǿ�������Ӷ�����̼��Ũ�ȣ�
��ת���ʣ�������ѹǿ�������Ӷ�����̼��Ũ�ȣ�
(4)![]()
![]() ��̼�Ļ��ϼ۴�+4��Ϊ-2��ÿ��̼ԭ�ӵõ�6�����ӣ���
��̼�Ļ��ϼ۴�+4��Ϊ-2��ÿ��̼ԭ�ӵõ�6�����ӣ���![]() ���õ�12n���ӣ����ݵ���غ�ɵøõ缫��ӦʽΪ��
���õ�12n���ӣ����ݵ���غ�ɵøõ缫��ӦʽΪ��![]()
![]()
![]() ��
��

 �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д� ��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
��Ԫ����ĩ��ϰ�ȷ��ϵ�д�