��Ŀ����
8���л���Y����ȡ������֬���{���������͵���Ҫԭ�ϣ�PVAc��֬����������Ϳ����PVA���л���N���������϶���������㾫������������Ǻϳ�·�����£�
��֪��RΪ������R�䡢R��Ϊ��������ԭ��
��R��CHO+R��CH2CHO$\stackrel{OH-}{��}$
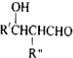 $\stackrel{��}{��}$
$\stackrel{��}{��}$
��RMgCl+
 ��
��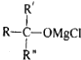 $\stackrel{H_{2}O}{��}$
$\stackrel{H_{2}O}{��}$
�ش��������⣺
��1��C����������ȩ
��2��д����B��һ������������PVAc��֬�Ļ�ѧ����ʽ��
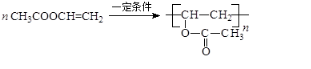
��3��D�Ľṹ��ʽ��CH3CH=CHCHO
��4��д��E��F�Ļ�ѧ����ʽ2CH3CH2CH2CH2OH+O2$��_{��}^{Cu}$2CH3CH2CH2CHO+2H2O
��5��д��F��G�Ļ�ѧ����ʽ2HCHO+CH3CH2CH2CHO$\stackrel{OH-}{��}$
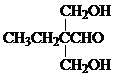
��6��M�Ľṹ��ʽ��
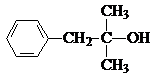
��7��A��B�ķ�Ӧ���ͼӳɷ�Ӧ
��8�������й�˵������ȷ����acd������ĸ��ţ�
a��N������������
b��C��K��Ϊͬϵ��
c��һ�������£�PVAc������NaOH��Һ��Ӧ
d����Ӧ�١��ڶ���������H2�ļӳɷ�Ӧ
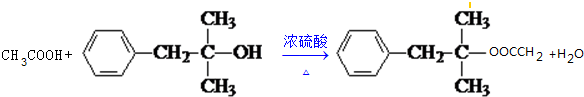
��9��X��M��Ӧ�Ļ�ѧ����ʽ
��10��д����������������B��ͬ���칹��Ľṹ��ʽ

a����B����ͬ�Ĺ����� b����ʽ�ṹ��
���� ����ϢI��Y�Ľṹ�����ƿ�֪GΪ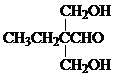
 ����N�ķ���ʽ��֪��������M����������Ӧ����N����M�ķ���ʽΪ��C12H16O2+H2O-C2H4O2=C10H14O����N�ķ����к���3��������M�к���2�����������ϢII��֪��KΪ
����N�ķ���ʽ��֪��������M����������Ӧ����N����M�ķ���ʽΪ��C12H16O2+H2O-C2H4O2=C10H14O����N�ķ����к���3��������M�к���2�����������ϢII��֪��KΪ ��MΪ
��MΪ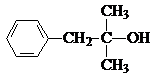
 ���ݴ˷������
���ݴ˷������
��� �⣺����ϢI��Y�Ľṹ�����ƿ�֪GΪ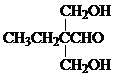
 ����N�ķ���ʽ��֪��������M����������Ӧ����N����M�ķ���ʽΪ��C12H16O2+H2O-C2H4O2=C10H14O����N�ķ����к���3��������M�к���2�����������ϢII��֪��KΪ
����N�ķ���ʽ��֪��������M����������Ӧ����N����M�ķ���ʽΪ��C12H16O2+H2O-C2H4O2=C10H14O����N�ķ����к���3��������M�к���2�����������ϢII��֪��KΪ ��MΪ
��MΪ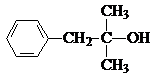
 ��
��
��1��������������֪��CΪCH3CHO����������ȩ���ʴ�Ϊ����ȩ��
��2����B��һ������������PVAc��֬�Ļ�ѧ����ʽ�� ��
��
�ʴ�Ϊ�� ��
��
��3��������������֪��D�Ľṹ��ʽ��CH3CH=CHCHO��
�ʴ�Ϊ��CH3CH=CHCHO��
��4��E��F�Ļ�ѧ����ʽΪ��2CH3CH2CH2CH2OH+O2$��_{��}^{Cu}$2CH3CH2CH2CHO+2H2O��
�ʴ�Ϊ��2CH3CH2CH2CH2OH+O2$��_{��}^{Cu}$2CH3CH2CH2CHO+2H2O��
��5��F��G�Ļ�ѧ����ʽΪ��2HCHO+CH3CH2CH2CHO$\stackrel{OH-}{��}$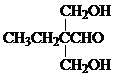
�ʴ�Ϊ��2HCHO+CH3CH2CH2CHO$\stackrel{OH-}{��}$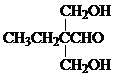
��6��������������֪��M�Ľṹ��ʽ��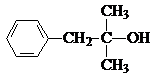
�ʴ�Ϊ��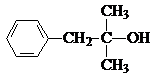
��7��ͨ�����Ϸ���֪��A����B�ķ�Ӧ�Ǽӳɷ�Ӧ���ʴ�Ϊ���ӳɷ�Ӧ��
��8�������й�˵������ȷ���� ������ĸ��ţ���
a��NΪ �����������������������ʣ���a��ȷ��
�����������������������ʣ���a��ȷ��
b��CΪCH3CHO������ȩ��KΪ ������ͪ�����߲�����ͬϵ���b����
������ͪ�����߲�����ͬϵ���b����
c��PVAc��֬Ϊ ������������һ�������£�������NaOH��Һ��Ӧ����c��ȷ��
������������һ�������£�������NaOH��Һ��Ӧ����c��ȷ��
d��������������֪����Ӧ�١��ڶ���������H2�ļӳɷ�Ӧ����d��ȷ��
��ѡ��acd��
��9���÷�Ӧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��10����������������B��CH3COOCH=CH2����ͬ���칹�壺a����B����ͬ�Ĺ����ţ�����������̼̼˫����b����ʽ�ṹ������������ͬ���칹��Ϊ��
�ʴ�Ϊ��
���� ���⿼���л����ƶϣ�Ϊ��Ƶ���㣬���ؿ���ѧ�������ƶ�������֪ʶǨ������������ijЩ���ʽṹ��ʽ����Ӧ�����������Ϣ�����ƶϣ���ȷ�����ż������ʹ�ϵ�ǽⱾ��ؼ�����Ŀ�ѶȲ���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | CH3-CH=CH2�� | B�� | 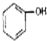 �� �� | C�� |  �� ��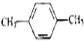 | D�� | ��������� |
| A�� | ����NH4HSO4���壬v��H2������ | B�� | ��������ˮ��v��H2����С | ||
| C�� | ����CH3COONa���壬v��H2����С | D�� | �μ�����CuSO4��Һ��v��H2���ӿ� |
��C��N��O��Al��Si��Cu�dz���������Ԫ�أ�
��1��Siλ��Ԫ�����ڱ��������ڵ�IVA�壮
��2��N�Ļ�̬ԭ�Ӻ�������Ų�ʽΪ1s22s22p3��Cu�Ļ�̬ԭ���������1�����ӣ�
��3���á�����������գ�
| ԭ�Ӱ뾶 | �縺�� | �۵� | �е� |
| Al��Si | N��O | ���ʯ������� | CH4��SiH4 |
��4��H2O2�ĵ���ʽ
 ��
����5��þȼ�ղ�����CO2����û�ѧ����ʽ��ʾ������2Mg+CO2$\frac{\underline{\;��ȼ\;}}{\;}$2MgO+C��
��6����AgCl�����м���KBr��Һ����ɫ����ת��Ϊ����ɫ������д����Ӧ�����ӷ���ʽAgCl��s��+Br-�TAgBr��s��+Cl-��
��7���������������ԭ��Ӧ�����ӷ���ʽ��
2MnO4-+5C2O42-+16H+=2Mn2++10CO2��+8H2O��
| A�� | ʯ���ѻ��ɻ����ϩ����ϩ�Ͷ���ϩ | |
| B�� | ˮ���������������ϼ��ͷ���� | |
| C�� | �������ᴿ�����ʲ��������������� | |
| D�� | ��������������������Ʒ�Ļ���ԭ�� |
| A�� | Na��Mg��Alԭ�������������������� | |
| B�� | N��O��FԪ����������������� | |
| C�� | Na��K��Rb�縺����С | |
| D�� | P��S��ClԪ����ۺ���������������ǿ |
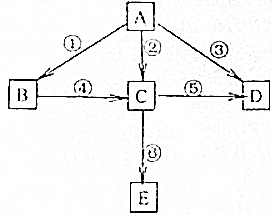 ��֪AΪ���ʣ�B��C��D��EΪ���������֮�������ͼת����ϵ��
��֪AΪ���ʣ�B��C��D��EΪ���������֮�������ͼת����ϵ��