��Ŀ����
���ⶨijNaOH��Һ�����ʵ���Ũ�ȣ�����0.1000mol?L-1HCl����Һ�����к͵ζ����÷�̪��ָʾ������
��ش��������⣺
��1������ѧ����ʵ������У���¼�ζ�ǰ�ζ�����Һ�����Ϊ1.10mL���ζ���Һ������ͼ�����ʱ���ı���Һ�����Ϊ______��
��2����ѧ����������ƽ��ʵ�飬���ݼ�¼���£�
ѡȡ�����������ݣ����������NaOH��Һ�����ʵ���Ũ��Ϊ______��С���������λ����
��3��������Щ������ʹ�ⶨ���ƫ��______������ţ���
A����ƿ������ˮϴ�������ô���Һ��ϴ
B����ʽ�ζ���������ˮϴ�������ñ�Һ��ϴ
C���ζ�ǰ��ʽ�ζ��ܼ������δ�ų����ζ���������ʧ
D���ζ�ǰ������ȷ���ζ����ӵζ��ܶ���
��4����˹�����������Ϳ�ѧ�о����к���Ҫ�����壮��Щ��Ӧ�ķ�Ӧ����Ȼ��ֱ�Ӳ�ã�����ͨ����ӵķ����ⶨ���ָ�������3���Ȼ�ѧ��Ӧ����ʽ��
��Fe2O3��s��+3CO��g���T2Fe��s��+3CO2��g����H=-24.8kJ?mol-1
��3Fe2O3��s��+CO��g���T2Fe3O4��s��+CO2��g����H=-47.2kJ?mol-1
��Fe3O4��s��+CO��g���T3FeO��s��+CO2��g����H=+640.5kJ?mol-1
д��CO���廹ԭFeO����õ�Fe�����CO2������Ȼ�ѧ��Ӧ����ʽ��______��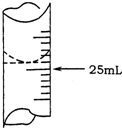
��ش��������⣺
��1������ѧ����ʵ������У���¼�ζ�ǰ�ζ�����Һ�����Ϊ1.10mL���ζ���Һ������ͼ�����ʱ���ı���Һ�����Ϊ______��
��2����ѧ����������ƽ��ʵ�飬���ݼ�¼���£�
| ʵ����� | ����NaOH��Һ�����/mL | 0.1000mol?L-1HCl��Һ�����/mL | |
| �ζ�ǰ�̶� | �ζ���̶� | ||
| 1 | 25.00 | 0.00 | 26.11 |
| 2 | 25.00 | 1.56 | 31.30 |
| 3 | 25.00 | 0.22 | 26.31 |
��3��������Щ������ʹ�ⶨ���ƫ��______������ţ���
A����ƿ������ˮϴ�������ô���Һ��ϴ
B����ʽ�ζ���������ˮϴ�������ñ�Һ��ϴ
C���ζ�ǰ��ʽ�ζ��ܼ������δ�ų����ζ���������ʧ
D���ζ�ǰ������ȷ���ζ����ӵζ��ܶ���
��4����˹�����������Ϳ�ѧ�о����к���Ҫ�����壮��Щ��Ӧ�ķ�Ӧ����Ȼ��ֱ�Ӳ�ã�����ͨ����ӵķ����ⶨ���ָ�������3���Ȼ�ѧ��Ӧ����ʽ��
��Fe2O3��s��+3CO��g���T2Fe��s��+3CO2��g����H=-24.8kJ?mol-1
��3Fe2O3��s��+CO��g���T2Fe3O4��s��+CO2��g����H=-47.2kJ?mol-1
��Fe3O4��s��+CO��g���T3FeO��s��+CO2��g����H=+640.5kJ?mol-1
д��CO���廹ԭFeO����õ�Fe�����CO2������Ȼ�ѧ��Ӧ����ʽ��______��

��1����¼�ζ�ǰ�ζ�����Һ�����Ϊ1.10mL���ζ���Һ������ͼΪ24.80ml���ζ����е�Һ�����Ϊ24.80ml-1.100ml=23.70mL��
�ʴ�Ϊ��23.70mL��
��2����Һ���ĵ����V����Һ��=
=27.31ml������c�����⣩=
=
=0.1092mol?L-1��
�ʴ�Ϊ��0.1092mol?L-1��
��3��A����ƿ������ˮϴ�������ô���Һ��ϴ������Һ���࣬���ı�Һ���࣬���ƫ�ߣ���A���ϣ�
B����ʽ�ζ���������ˮϴ�������ñ�Һ��ϴ������ʵ�������������ϣ���B�����ϣ�
C���ζ�ǰ��ʽ�ζ��ܼ������δ�ų����ζ���������ʧ�������ı�Һ��������ƫ�ߣ���C���ϣ�
D���ζ�ǰ������ȷ���ζ����ӵζ��ܶ�����������С����Һ�����С�����ƫ�ͣ���D�����ϣ�
�ʴ�Ϊ��AC��
��4����Fe2O3��s��+3CO��g���T2Fe��s��+3CO2��g����H=-24.8kJ?mol-1
��3Fe2O3��s��+CO��g���T2Fe3O4��s��+CO2��g����H=-47.2kJ?mol-1
��Fe3O4��s��+CO��g���T3FeO��s��+CO2��g����H=+640.5kJ?mol-1
���ݸ�˹���ɢ١�3-�ۡ�2+�ڵõ���6CO��g��+6FeO��s��=6Fe��s��+6CO2��g����H=-1242.2KJ/mol��
�õ��Ȼ�ѧ����ʽΪ��CO��g��+FeO��s��=Fe��s��+CO2��g����H=-207.0KJ/mol��
�ʴ�Ϊ��CO��g��+FeO��s��=Fe��s��+CO2��g����H=-207.0KJ/mol��
�ʴ�Ϊ��23.70mL��
��2����Һ���ĵ����V����Һ��=
| 26.11+31.30-1.56+26.31-0.22 |
| 3 |
| c(��Һ)��V(��Һ) |
| V(����Һ) |
| 0.1000mol/L��0.0273L |
| 0.0250L |
�ʴ�Ϊ��0.1092mol?L-1��
��3��A����ƿ������ˮϴ�������ô���Һ��ϴ������Һ���࣬���ı�Һ���࣬���ƫ�ߣ���A���ϣ�
B����ʽ�ζ���������ˮϴ�������ñ�Һ��ϴ������ʵ�������������ϣ���B�����ϣ�
C���ζ�ǰ��ʽ�ζ��ܼ������δ�ų����ζ���������ʧ�������ı�Һ��������ƫ�ߣ���C���ϣ�
D���ζ�ǰ������ȷ���ζ����ӵζ��ܶ�����������С����Һ�����С�����ƫ�ͣ���D�����ϣ�
�ʴ�Ϊ��AC��
��4����Fe2O3��s��+3CO��g���T2Fe��s��+3CO2��g����H=-24.8kJ?mol-1
��3Fe2O3��s��+CO��g���T2Fe3O4��s��+CO2��g����H=-47.2kJ?mol-1
��Fe3O4��s��+CO��g���T3FeO��s��+CO2��g����H=+640.5kJ?mol-1
���ݸ�˹���ɢ١�3-�ۡ�2+�ڵõ���6CO��g��+6FeO��s��=6Fe��s��+6CO2��g����H=-1242.2KJ/mol��
�õ��Ȼ�ѧ����ʽΪ��CO��g��+FeO��s��=Fe��s��+CO2��g����H=-207.0KJ/mol��
�ʴ�Ϊ��CO��g��+FeO��s��=Fe��s��+CO2��g����H=-207.0KJ/mol��

��ϰ��ϵ�д�
 ��һ����ͬ���ɽ�����ϵ�д�
��һ����ͬ���ɽ�����ϵ�д� ������Ӧ���ϵ�д�
������Ӧ���ϵ�д�
�����Ŀ
 ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�
ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£� ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�
ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£� ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�
ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£� ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�
ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£� ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�
ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�