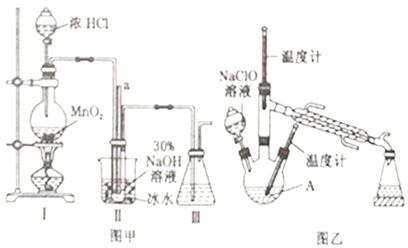
��Ŀ����
����Ŀ����ҵ���ú��̷���(��Ҫ�ɷ�MnO2����������Fe2O3��Al2O3��CuO��CaO��)����������������ϴ������Ʊ�MnSO4���������£�
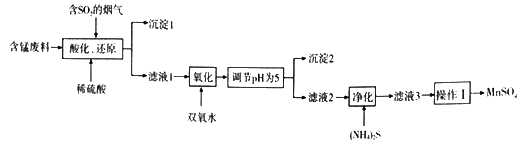
��֪��25��ʱ����������������ܶȻ�����(Ksp)���±���ʾ��
�������� | Al(OH)3 | Fe(OH)3 | Cu(OH)2 | Mn(OH)2 |
Ksp | 1.3��10-33 | 4.0��10-38 | 2.2��10-20 | 1.9��10-14 |
��ش�
(1)����1�Ļ�ѧʽΪ__________________��
(2)�����£�����pHΪ5.��ͨ������˵����ʱAl3+��Fe3+�ѳ�����ȫ��������_________��(NH4)2S�ĵ���ʽΪ________________����������ʱ������(NH4)2S������Ϊ___________________��
(3)���ữ����ԭ���У�����������������ԭ��Ӧ�����ӷ���ʽΪ__________________��
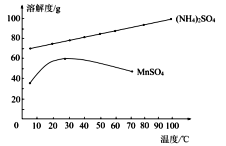
(4)��֪����Һ3�г�MnSO4�⣬����������(NH4)2SO4��(NH4)2SO4��MnSO4���ܽ����������ͼ��ʾ��
�ݴ��жϣ�������I��ӦΪ����Ũ����____________��ϴ�ӡ����
(5)��ҵ�Ͽ��õ������MnSO4��Һ�ķ����Ʊ�MnO2����������ӦʽΪ________________��
(6)25.35 g MnSO4��H2O��Ʒ���ȷֽ���̵���������(��Ʒ�������¶ȱ仯������)����ͼ��ʾ��
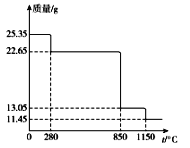
��300��ʱ�����ù���Ļ�ѧʽΪ______________________��
��1150��ʱ����Ӧ�Ļ�ѧ����ʽΪ___________________��
���𰸡� CaSO4 pH=5ʱ��c(OH-)=10-9mol/L��c(Fe3+)=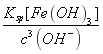 =
=![]() =4.0��10-11mol/L��ͬ����c(Al3+)=1.3��10-6mol/L����С��1.0��10-5mol/L����Al3+��Fe3+�ѳ�����ȫ
=4.0��10-11mol/L��ͬ����c(Al3+)=1.3��10-6mol/L����С��1.0��10-5mol/L����Al3+��Fe3+�ѳ�����ȫ 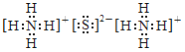 ʹCu2+ת��ΪCuS���� MnO2+SO2��Mn2++SO42- ��Fe2O3+SO2+2H+��2Fe2++SO42-+H2O ���ȹ��� Mn2++2H2O-2e-=MnO2+4H+ MnSO4 3MnO2
ʹCu2+ת��ΪCuS���� MnO2+SO2��Mn2++SO42- ��Fe2O3+SO2+2H+��2Fe2++SO42-+H2O ���ȹ��� Mn2++2H2O-2e-=MnO2+4H+ MnSO4 3MnO2 ![]() Mn3O4��O2��
Mn3O4��O2��
�����������̷���(��Ҫ�ɷ�MnO2����������Fe2O3��Al2O3��CuO��CaO��)�뺬�ж�������������ϡ�����ܽ⣬����SO2+MnO2�TMn2++SO42-��Fe2O3+SO2+2H+=2Fe2++SO42-+H2O��Al2O3+6H+=2Al3++3H2O��CuO+2H+=Cu2++H2O��CaO+2H++SO42-=CaSO4��+H2O������1ΪCaSO4����Һ1 ����������ΪMn2+��Fe2+��Al3+��Cu2+������˫��ˮ����Fe2+ΪFe3+������pHΪ5����Fe3+��Al3+������2ΪAl(OH)3��Fe(OH)3����Һ2����������ΪMn2+��Cu2+������(NH4)2S����Cu2+����Һ3�г�MnSO4�⣬����������(NH4)2SO4������Һ3����Ũ�������ȹ��ˡ�ϴ�ӡ������Ʊ�MnSO4��
(1)���̷�����ϡ���ᷴӦ��CaO+2H++SO42-=CaSO4��+H2O������1ΪCaSO4���ʴ�Ϊ��CaSO4��
(2) pH=5ʱ��c(OH-)=10-9 mol/L��c(Fe3+)=![]() =
=![]() =4.0��10-11 mol/L��ͬ����c(Al3+)=1.3��10-6 mol/L����С��1.0��10-5 mol/L����Al3+��Fe3+�ѳ�����ȫ��(NH4)2SΪ���ӻ��������ʽΪ��
=4.0��10-11 mol/L��ͬ����c(Al3+)=1.3��10-6 mol/L����С��1.0��10-5 mol/L����Al3+��Fe3+�ѳ�����ȫ��(NH4)2SΪ���ӻ��������ʽΪ��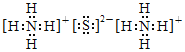 ��(NH4)2S�����ó���Cu2+ʹCu2+ת��ΪCuS�������ʴ�Ϊ��pH=5ʱ��c(OH-)=10-9 mol/L��c(Fe3+)=
��(NH4)2S�����ó���Cu2+ʹCu2+ת��ΪCuS�������ʴ�Ϊ��pH=5ʱ��c(OH-)=10-9 mol/L��c(Fe3+)=![]() =
=![]() =4.0��10-11 mol/L��ͬ����c(Al3+)=1.3��10-6 mol/L����С��1.0��10-5 mol/L����Al3+��Fe3+�ѳ�����ȫ��
=4.0��10-11 mol/L��ͬ����c(Al3+)=1.3��10-6 mol/L����С��1.0��10-5 mol/L����Al3+��Fe3+�ѳ�����ȫ��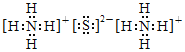 ��ʹCu2+ת��ΪCuS������
��ʹCu2+ת��ΪCuS������
(3)���ữ����ԭ���У�����������������ԭ��Ӧ�����ӷ���ʽΪ��SO2+MnO2�TMn2++SO42-��Fe2O3+SO2+2H+=2Fe2++SO42-+H2O���ʴ�Ϊ��SO2+MnO2�TMn2++SO42-��Fe2O3+SO2+2H+=2Fe2++SO42-+H2O��
(4)��ͼ��֪(NH4)2SO4���ܽ�����¶ȵ����߶�����MnSO4���ܽ�����¶ȵ�����С���ʽ���Һ3����Ũ�������ȹ��ˡ�ϴ�ӡ������Ʊ�MnSO4���ʴ�Ϊ�����ȹ��ˣ�
(5)�õ������MnSO4��Һ�ķ����Ʊ�MnO2����������������Ӧ����ӦʽΪ��Mn2++2H2O-2e-=MnO2+4H+���ʴ�Ϊ��Mn2++2H2O-2e-=MnO2+4H+��
(6)��25.35gMnSO4H2O��Ʒn(��)=n(MnSO4H2O)=0.15mol������n(H2O)=0.15mol��m(H2O)=2.7g��300��ʱ�����ù�������Ϊ22.65g�����ٵ�����Ϊ2.7g����˵���ö�ʧȥ�ᾧˮ����ʱ����Ϊ��MnSO4���ʴ�Ϊ��MnSO4��
��300��ʱ������Ϊ��MnSO4�����ȷֽ������̵�����������������0.15mol��850��ʱ������������22.65g���ٵ�Ϊ13.05g�����ٵ�����Ϊ9.6g���������������������Ϊ64����Ϊ�����������ʱ�Ĺ���ΪMnO2��1150��ʱ����Ϊ�������̷ֽ����ã���Ԫ�������غ㣬��m(��)=n(��)��55=8.25g������������m(O)=11.45g-8.25g=3.2g��n(O)=0.2mol����n(Mn)��n(O)=0.15��0.2=3��4�����������Ϊ��Mn3O4���ʷ�ӦΪ��3MnO2![]() Mn3O4+O2�����ʴ�Ϊ��3MnO2
Mn3O4+O2�����ʴ�Ϊ��3MnO2![]() Mn3O4+O2����
Mn3O4+O2����

 ѧ���쳵�����ּ��ں�����ҵϵ�д�
ѧ���쳵�����ּ��ں�����ҵϵ�д�����Ŀ������һƿA��B�Ļ��Һ����֪A��B��ijЩ�������£�
���� | ����ʽ | �۵�� | �е�� | �ܶ�gcm-3 | ˮ���� |
A | C3H6O2 | -98 | 57.5 | 0.93 | ���� |
B | C4H8O2 | -84 | 77 | 0.90 | ���� |
�ɴˣ�����A��B����ѷ����� �� ��
A. ��ȡ B. ���� C. ���� D. ��Һ