��Ŀ����
��15�֣��Ҵ������ᶼ���л�������Ҫ�Ļ���ԭ�ϡ�
��1���������У��Ҵ�������ʹ����ͭ˿���ֺ��ȣ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��2����Ũ������£�������Ҵ�������������

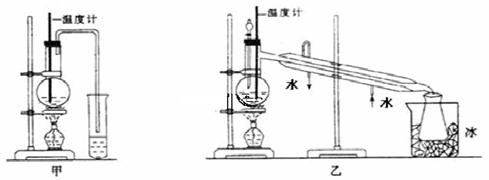
ij��ѧ��ȤС���ͬѧ������װ�ý��и�������Ӧ��̽��ʵ�飺
��Ҫ����Թ��м�2mLŨ���ᡢ3mL�Ҵ���2mL���ᣬ���������� ��
��С�Թ���װ����ŨNa2CO3��Һ�������ܲ�����Һ������Ϊ�˷�ֹ ��
��������ʵIJ������ʣ�
| | �Ҵ� | ���� | �������� |
| �е� | 78��0�� | 117��9�� | 77��5�� |
| ˮ���� | ���� | ���� | ���� |
�ݷ���ʱ����������Ӧ�ô����� ����¿ڷš� ���Ͽڵ���������
����ȤС�����������Ҵ����������������õ��������������������±���
| ʵ����� | �Ҵ���mL�� | ���ᣨmL�� | ����������mL�� |
| a | 2 | 2 | 1��33 |
| b | 3 | 2 | 1��57 |
| c | 4 | 2 | X |
| d | 5 | 2 | 1��76 |
| e | 2 | 3 | 1��55 |
��2CH3CH2OH + O2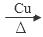 2CH3CHO + 2H2O
2CH3CHO + 2H2O
��2���ٴ��Թ��м���3mL�Ҵ���Ȼ������Թܱ���2mLŨ�����2mL���ᡣ�ڵ�����
�۲��ܣ������������Ҵ��������ˮ�Ļ����Һ���ܽ�ȱȽϴܷ�Һ©�� ���Ͽڵ���
��1��57-1��76mL�� ̽��������������������������Ӱ�졣
���������������1�������У��Ҵ�������ʹ����ͭ˿���ֺ��ȣ��÷�Ӧ�Ļ�ѧ����ʽΪ2CH3CH2OH + O2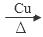 2CH3CHO + 2H2O; (2)��Ҫ����Թ��м�2mLŨ���ᡢ3mL�Ҵ���2mL���ᣬ����������������Թ��м���3mL�Ҵ���Ȼ������Թܱ���2mLŨ���ᣬ����Һ��ȴ���ټ���2mL���ᡣ��С�Թ���װ����ŨNa2CO3��Һ�������������ջӷ����Ҵ���������Ӧ�����������������ᣬ��������ζ�IJ��������������������ܽ�ȣ��Ա��ڻ����ķ����ᴿ�������ܲ�����Һ������Ϊ�˷�ֹ��������ķ������۲�����ˮ���С�Թ��е�Na2CO3��Һ������Ϊ�����������Ҵ��������ˮ�Ļ����Һ���ܽ�ȱȽϴܷ���С�Թ��л������ܵ�����Һ������ķ����Ƿ�Һ��ʹ�õ������Ƿ�Һ©�������ڷ���ʱ�����������������ܶȱ�ˮС�����ϲ㣬ʹ��Ӧ�ô������Ͽڵ��������ݱ������ݱ仯���ɿ�֪����������X�ķ�Χ��1��57-1��76mL��ʵ��a��ʵ��e���Ҵ���������ͬ��������������ͬ���Ҵ�������ʹ��̽����Ŀ����̽��������������������������Ӱ�졣
2CH3CHO + 2H2O; (2)��Ҫ����Թ��м�2mLŨ���ᡢ3mL�Ҵ���2mL���ᣬ����������������Թ��м���3mL�Ҵ���Ȼ������Թܱ���2mLŨ���ᣬ����Һ��ȴ���ټ���2mL���ᡣ��С�Թ���װ����ŨNa2CO3��Һ�������������ջӷ����Ҵ���������Ӧ�����������������ᣬ��������ζ�IJ��������������������ܽ�ȣ��Ա��ڻ����ķ����ᴿ�������ܲ�����Һ������Ϊ�˷�ֹ��������ķ������۲�����ˮ���С�Թ��е�Na2CO3��Һ������Ϊ�����������Ҵ��������ˮ�Ļ����Һ���ܽ�ȱȽϴܷ���С�Թ��л������ܵ�����Һ������ķ����Ƿ�Һ��ʹ�õ������Ƿ�Һ©�������ڷ���ʱ�����������������ܶȱ�ˮС�����ϲ㣬ʹ��Ӧ�ô������Ͽڵ��������ݱ������ݱ仯���ɿ�֪����������X�ķ�Χ��1��57-1��76mL��ʵ��a��ʵ��e���Ҵ���������ͬ��������������ͬ���Ҵ�������ʹ��̽����Ŀ����̽��������������������������Ӱ�졣
���㣺�����Ҵ��Ĵ�����������������ʵ������ȡ��Ӧ����Ӧԭ���������ķ����֪ʶ��

 ��У����ϵ�д�
��У����ϵ�д���5�֣������м������ʣ��뽫����������пո��ڣ�
| A��CH2=CH-COOH�����ᣨC17H33COOH�� |
| B��12C60��ʯī |
C�� �� ��  |
| D��35Cl��37ClE���Ҵ����Ҷ��� |
�ۻ�Ϊͬ����������� ���ܻ�Ϊͬ���칹����� ��
�ݼȲ���ͬϵ��ֲ���ͬ�����壬Ҳ����ͬ�������壬���ɿ�����ͬһ�����ʵ���
ʵ������ȡ���ᶡ����ʵ��װ����������ͼ��ʾ����װ�ù�ѡ�á����й����ʵ���������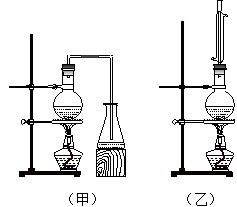
���±���
| | ���� | 1-���� | ���ᶡ�� |
| �۵�(��) | 16.6 | ��89.5 | ��73.5 |
| �е�(��) | 117.9 | 117 | 126.3 |
| �ܶ�(g/cm3) | 1.05 | 0.81 | 0.88 |
| ˮ���� | ���� | ����(9g/100gˮ) | �� |
��2����ʵ���������г������������ᶡ���⣬���������ɵ��л��������У�д���ṹ��ʽ���� �� ��
��3��������Ӧ��һ�����淴Ӧ��Ϊ���1-�����������ʣ��ɲ�ȡ�Ĵ�ʩ�� ��
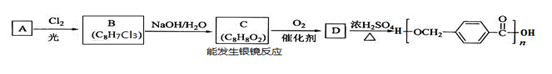
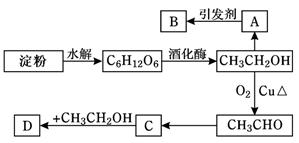
��4�����Ʊ����ᶡ�����õĻ�����з��롢�ᴿ���ᶡ��ʱ����Ҫ�����ಽ����������ͼʾ�IJ����У��϶���Ҫ�Ļ�ѧ������___________��ѡ��𰸱�ţ���
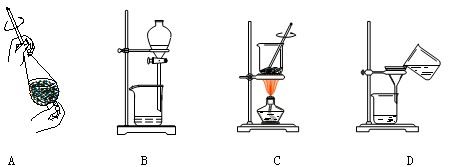
��5���л���ķ�������У�������Ҫʹ�÷�Һ©����������ʹ�÷�Һ©��ǰ���� ��ijͬѧ�ڽ��з�Һ����ʱ��������Һ�����������������ԭ�����Һ©�����������⣬������ ��




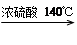 H2O + CH3CH2��O��CH2CH3 (����)
H2O + CH3CH2��O��CH2CH3 (����)