��Ŀ����
ʵ����ģ���ù�ҵ�������壨����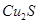 ��Al2O3��Fe2O3��SiO2�ȣ���ȡ��ͭ���̷���
��Al2O3��Fe2O3��SiO2�ȣ���ȡ��ͭ���̷���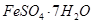 ����������Kal��SO4��2
����������Kal��SO4��2 12H2O�ݵIJ����������£�
12H2O�ݵIJ����������£�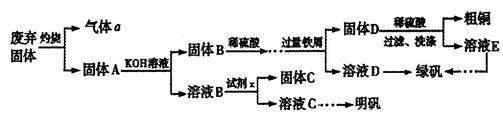
��1���Լ�x��_________��д��ѧʽ����
��2����ƽ���з���ʽ��
____
��4��Ϊ�˷�����Ʒ���̷�������Ԫ�صĺ�����ijͬѧ��ȡ20.0g��Ʒ���100mL��Һ����ȡ25.00mL��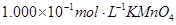 ����Һ���еζ���MnO
����Һ���еζ���MnO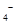 ����ԭΪ
����ԭΪ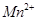 ����
����
��ش��������⣺
�ٵζ�ʱ��KmnO4����ҺӦʢ����______________�����������ƣ��С�
�����ﵽ�ζ��յ�����KmnO4����Һ���Ϊ25.00mL����ò�Ʒ����Ԫ�ص���������Ϊ_________��
��1��H2SO4����KHSO4����2�֣�
��2������Ũ���������Ũ������Ũ��������ȴ�ᾧ��4�֣�
��3��2��5��2��2��5��4��3�֣�
��4������ʽ�ζ��ܣ�2�֣� ��14%��3�֣�
���������������1������KOH����ҺB����K[Al(OH)4],��������Ϊ����������XΪH2SO4����KHSO4����
��3�����ݻ��ϼ�������������ƽ��ѧ����ʽ��
��4���ٸ�����ؾ���ǿ�����ԣ��������ܣ�ֻ��ʢ������ʽ�ζ����У�
�ڸ��ݵ����غ��֪��KMnO4��5Fe2+,20.0��Ʒ�к�����0.025L��0.1mol/L��5��4��56g/mol=2.8g�����������Ԫ�ص�����������
���㣺���⿼��������ͼ���������ӷ���ʽ����ƽ���ζ�����ѧ���㡣

�������ȣ�ClO2��Ϊһ�ֻ���ɫ���壬�ǹ����Ϲ��ϵĸ�Ч�����ס����١���ȫ��ɱ����������
��1����ҵ���Ʊ�ClO2�ķ�Ӧԭ�������ã�2NaClO3��4HCl=2ClO2����Cl2����2H2O��2NaCl��
��Ũ�����ڷ�Ӧ����ʾ������������_______������ţ���
| A��ֻ�л�ԭ�� | B����ԭ�Ժ����� | C��ֻ�������� | D�������Ժ����� |
��2��Ŀǰ�ѿ������õ�ⷨ��ȡClO2���¹��ա�
����ͼʾ����ʯī���缫����һ�������µ�ⱥ��ʳ��ˮ��ȡClO2��д����������ClO2�ĵ缫��Ӧʽ�� ��
�ڵ��һ��ʱ�䣬�������������������Ϊ112 mL����״����ʱ��ֹͣ��⡣ͨ�������ӽ���Ĥ�������ӵ����ʵ���Ϊ_________mol����ƽ���ƶ�ԭ������������pH�����ԭ�� ��
��3��ClO2����ˮ��Fe2����Mn2����S2����CN���������Ե�ȥ��Ч����ij������ˮ�к�
CN�� a mg/L������ClO2��CN-������ֻ�����������壬�����ӷ�Ӧ����ʽΪ ��
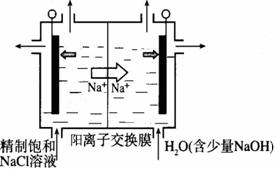
Ϊ��֤������Cl2��Fe3+��SO2��ijС������ͼ��ʾװ�ý���ʵ�飨�г������� A�м���װ�����ԣ��������Ѽ��飩��
ʵ����̣�
��.���ɼ�K1~K4��ͨ��һ��ʱ��N2���ٽ�T�͵��ܲ���B�У�����ͨ��N2��Ȼ��ر�K1��K3��K4��
��.����a���μ�һ������Ũ���ᣬ��A���ȡ�
��.��B����Һ���ʱ��ֹͣ���ȣ��н����ɼ�K2��
��.����b��ʹԼ2mL����Һ����D�Թ��У��������е����ӡ�
��.���ɼ�K3������c������70%�����ᣬһ��ʱ���н����ɼ�K3��
��.�����Թ�D���ظ����̢�������B��Һ�е����ӡ�
��1��A�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2����70%��������ȡSO2����Ӧ���ʱ���98%������죬ԭ���� ��
��3���ס��ҡ�����λͬѧ�ֱ����������ʵ�飬���ǵļ����һ���ܹ�֤��������
Cl2��Fe3+��SO2���� ����ס����ҡ�����������
| | ���̢� B��Һ�к��е����� | ���̢� B��Һ�к��е����� |
| �� | ��Fe3+��Fe2+ | ��SO42- |
| �� | ����Fe3+����Fe2+ | ��SO42- |
| �� | ��Fe3+��Fe2+ | ��Fe2+ |
��4������ʵ����̢�ʱ��B����Һ��ɫ�ɻ�ɫ��Ϊ����ɫ��ֹͣͨ��������һ��ʱ�����Һ��ɫ��Ϊdz��ɫ��
�������ϣ�Fe2+��aq����SO32-��aq��
 FeSO3��s����ī��ɫ��
FeSO3��s����ī��ɫ��������裺FeCl3�� SO2�ķ�Ӧ�������м����FeSO3����Һ�ĺ���ɫ��FeSO3��ī��ɫ����FeCl3����ɫ���Ļ��ɫ��ijͬѧ�������ʵ�飬֤ʵ�ü��������
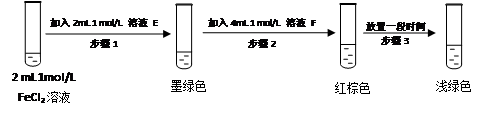
�� ��ҺE��F�ֱ�Ϊ �� ��
�� ���û�ѧƽ��ԭ�����Ͳ���3�к���ɫ��Һ��ɫ��Ϊdz��ɫ��ԭ��
��



 7N2+12H2O��
7N2+12H2O�� 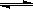 2SO3 (g)������H =��196.6kJ��mol��1
2SO3 (g)������H =��196.6kJ��mol��1