��Ŀ����
����Ŀ��ʯ���ǹ�ҵ��ѪҺ��ͨ�������Եõ��ܶ���Ҫ�Ļ�����Ʒ��

��֪��
![]()
 +
+
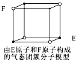
(1)B��AΪͬϵ�B�Ľṹ��ʽΪ_______��д��B�������ӳɺ�IJ�������______��
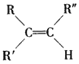
(2)��Ӧ�ڵĻ�ѧ����ʽΪ__________���䷴Ӧ����Ϊ_________��
(3)д������C3H5Cl�к���Clԭ�ӵķ���_____________��
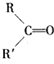
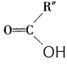
(4)C�Ľṹ��ʽΪ_______________��������ŵ�����Ϊ__________��
(5)��Ӧ�ܵĻ�ѧ����ʽΪ______________��
(6)ʯ���ѽ����л����б�Ȳ��Ϊ�о����ʵķ����ԣ��ɽ�CH![]() C-CH3���۵õ�����ͬϵ�����ܵĽṹ��ʽ��_______�֡�
C-CH3���۵õ�����ͬϵ�����ܵĽṹ��ʽ��_______�֡�
(7)���һ������ϩΪԭ���Ʊ�D�ĺϳ�·��________ (������ԭ����ѡ)���ϳ�·�߳��õı�ʾ��ʽΪ��A![]() B
B![]() Ŀ�����
Ŀ�����
���𰸡�CH2=CHCH3 1,2-���ȱ��� HOOC-COOH+2CH3CH2OH![]() CH3CH2OOCCOOCH2CH3+2H2O ������Ӧ ȡ������������������Һ�����ȣ���ȴ����ϡ�����ữ���ٵμ���������Һ�����ɰ�ɫ����˵����Clԭ�� HOOC-COOH �Ȼ� CH2=CHCH2Cl+NaOH
CH3CH2OOCCOOCH2CH3+2H2O ������Ӧ ȡ������������������Һ�����ȣ���ȴ����ϡ�����ữ���ٵμ���������Һ�����ɰ�ɫ����˵����Clԭ�� HOOC-COOH �Ȼ� CH2=CHCH2Cl+NaOH![]() CH2=CHCH2OH+NaCl 2
CH2=CHCH2OH+NaCl 2 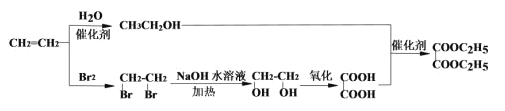
��������
C2H6O��C��Ӧ����D����D�Ľṹ��ʽ��֪CΪHOOC-COOH��C2H6OΪCH3CH2OH����AΪCH2=CH2����ˮ�����ӳɷ�Ӧ�����Ҵ���C3H5Cl����±������ˮ�ⷴӦ����CH2=CHCH2OH����C3H5ClΪCH2=CHCH2Cl��BΪCH2=CHCH3���ݴ˷������
(1)����B��������Ӧ����ΪC3H5Cl���÷�ӦΪȡ����Ӧ����B����ʽΪC3H6��B��AΪͬϵ���֪B�к���һ�������ͼ������ݷ���ʽ��֪BΪCH2=CHCH3��B�������ӳ�����CH2ClCHClCH3��������Ϊ1,2-���ȱ��飻
(2)��Ӧ��ΪHOOC-COOH��CH3CH2OH��������Ӧ������ʽΪHOOC-COOH+2CH3CH2OH![]() CH3CH2OOCCOOCH2CH3+2H2O��
CH3CH2OOCCOOCH2CH3+2H2O��
(3)����C3H5Cl����ԭ�ӿ�ȡ������������������Һ�����ȣ���ȴ����ϡ�����ữ���ٵμ���������Һ�����ɰ�ɫ����˵����Clԭ�ӣ�
(4)���ݷ�����֪CΪHOOC-COOH��������Ϊ�Ȼ���
(5)��Ӧ��Ϊ��ԭ�ӵ�ȡ����Ӧ������ʽΪCH2=CHCH2Cl+NaOH![]() CH2=CHCH2OH+NaCl��
CH2=CHCH2OH+NaCl��
(6)CH��C-CH3���۵õ�����ͬϵ�����ܽṹ��ʽΪ��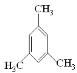 ��
��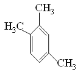 ���������֣�
���������֣�
(7)DΪ�Ҷ���������������Ҷ�����Ҵ�����������Ӧ���ɵģ���ϩ��ˮ�����ӳɷ�Ӧ�������Ҵ�����ϩ����ˮ�����ӳɷ�Ӧ����1��2-�������飬1��2-�����������������Ƶ�ˮ��Һ��ˮ�������Ҷ������Ҷ����������������Ҷ��ᣬ��������ϩΪԭ���Ʊ�D�ĺϳ�·��Ϊ ��
��

����Ŀ�������Ǵ����о��εġ��˹��̵������·�����N2�ڴ���������ˮ������Ӧ��2N2(g)+6H2O(l)![]() 4NH3(g)+3O2(g)-1530.4kJ�����������գ�
4NH3(g)+3O2(g)-1530.4kJ�����������գ�
(1)���Ȼ�ѧ��Ӧ����ʽ��������________________________________________��
(2)������Ӧ�ﵽƽ����������������䣬�����¶ȣ����´ﵽƽ��ʱ_______��
a��ƽ�ⳣ��K���� b��H2O��Ũ�ȼ�С
c�������ڵ�ѹǿ���� d��v��(O2)��С
(3)����ʵ�����ݼ��±���������2L�������������⡢N21mol��ˮ3mol����Ӧʱ��3 h����
��� | ��һ�� | �ڶ��� | ������ | ������ |
t/�� | 30 | 40 | 50 | 80 |
NH3������/��10��6mol�� | 4.8 | 5.9 | 6.0 | 2.0 |
������������3Сʱ����NH3��ʾ��ƽ����Ӧ������________________��������������NH3��������С�Ŀ���ԭ����_________________________________________��
(4)���ø÷�Ӧ���й�ҵ��������ѡ�����˵�����___________________________ ��
(5)�����������������أ��̬�����Լ����ᣬ�������������������(NH4)2SO4��Һ�д���ˮ�ⷴӦ�������¸�ˮ�ⷴӦ��ƽ�ⳣ������ʽ�ɱ�ʾΪK=_____________��
(6)�е�Ũ�ȵ�����������Һ����NH4Cl����(NH4)2SO4����NH4HSO4��c(NH4+)�ɴ�С��˳����____________________��������pHֵ��ȣ���������Һ�У�c(NH4+)�ɴ�С��˳����____________________��