��Ŀ����
��1��ij�о���ѧϰС����ʵ����������1mol/L��ϡ�������Һ��Ȼ������ζ�ijδ֪Ũ�ȵ�NaOH��Һ�������й�˵������ȷ����______��
A��ʵ�������õ��ĵζ��ܡ�����ƿ����ʹ��ǰ����Ҫ��©��
B�����ʵ��������60mL ��ϡ�������Һ������ʱӦѡ��100mL����ƿ��
C������ƿ�к�����������ˮ���ᵼ���������Һ��Ũ��ƫС��
D����ʽ�ζ���������ˮϴ�Ӻ�װ���Ũ�ȵ�ϡ���ᣬ���õ�NaOH��Һ��Ũ�Ƚ�ƫ��
E��������Һʱ���������һ�ζ���ʱ���Ӷ������������ʵ����ƫ��
F���к͵ζ�ʱ���������һ�ζ���ʱ���Ӷ������������ʵ����ƫ��
��2�������£���֪0.1mol?L-1һԪ��HA��Һ��c��OH-��/c��H+��=1��10-8��
�ٳ����£�0.1mol?L-1 HA��Һ��pH=______��д�����ᣨHA����NaOH��Һ��Ӧ�����ӷ���ʽ��______��
��pH=3��HA��pH=11��NaOH��Һ�������Ϻ���Һ��4���������ʵ���Ũ�ȴ�С��ϵ�ǣ�______��
��0.2mol?L-1HA��Һ��0.1mol?L-1NaOH��Һ�������Ϻ�������Һ�У�
c��H+��+c��HA��-c��OH-��=______mol?L-1������Һ����仯���Բ��ƣ�
��3��t��ʱ����pH=2��ϡ�����pH=11��NaOH��Һ�������Ϻ���Һ�����ԣ�����¶���ˮ�����ӻ�����Kw=______��
�ٸ��¶��£�t�棩����100mL 0.1mol?L-1��ϡH2SO4��Һ��100mL 0.4mol?L-1��NaOH��Һ��Ϻ���Һ����仯���Բ��ƣ�����Һ��pH=______��
�ڸ��¶��£�t�棩��1�����ϡ�����10�����NaOH��Һ��Ϻ���Һ�����ԣ���ϡ�����pH��pHa����NaOH��Һ��pH��pHb���Ĺ�ϵ�ǣ�______��
�⣺��1��A��ʵ�������õ��ĵζ��ܡ�����ƿ����ʹ��ǰ����Ҫ��©��������������A��ȷ��
B�����ʵ��������60mL ��ϡ�������Һ������ʱӦѡ��100mL����ƿ������ƿ�Ĺ����50mL��100mL������ƿ��Ӧѡ���Դ��ڻ����������Һ���������ƿ����B��ȷ��
C������ƿ�к�����������ˮ����Ӱ�����ʵ����ʵ�������Һ����������Բ��ᵼ���������Һ��Ũ��ƫС����C����
D����ʽ�ζ���������ˮϴ�Ӻ�װ���Ũ�ȵ�ϡ���ᣬ����ϡ�����Ũ��ƫС������ϡ��������ƫ�����Բ�õ�NaOH��Һ��Ũ�Ƚ�ƫ��D��ȷ��
E��������Һʱ���������һ�ζ���ʱ���Ӷ�����������Һ�����ƫСŨ��ƫ������������ƫС���ʵ���ʵ����ƫС����E����
F���к͵ζ�ʱ���������һ�ζ���ʱ���Ӷ�������������������ƫС���ʵ���ʵ����ƫС����F����
��ѡABD��
��2����c��OH-��/c��H+��=1��10-8��c��OH-����c��H+��=1.0��10-14����c��H+��=10-3mol?L-1��������Һ��pH=3��������Ũ��С�����Ũ�ȣ����Ը��������ᣬ���ᣨHA����NaOH��Һ��Ӧ�����ӷ���ʽΪ��HA+OH-�TA-+H2O��
�ʴ�Ϊ��3��HA+OH-�TA-+H2O��
��pH=11��NaOH��Һ��c��OH-��=10-3mol?L-1��HA�����ᣬ���Ũ��ԶԶ����������Ũ�ȣ�����pH=3��HA��pH=11��NaOH��Һ�������Ϻ���Һ�е�����������Σ���Һ�����ԣ�������Һ��������Ũ�ȴ�������������Ũ�ȣ��������Ũ�ȴ���������Ũ�ȣ���Һ��4���������ʵ���Ũ�ȴ�С��ϵ��c��A-����c��Na+����c��H+����c��OH-����
�ʴ�Ϊ��c��A-����c��Na+����c��H+����c��OH-����
��0.2mol?L-1HA��Һ��0.1mol?L-1NaOH��Һ�������Ϻ�������Һ�У��ɵ���غ��У�c��Na+��+c��H+��=c��A-��+c��OH-�����������غ��У�2c��Na+��=c��A-��+c��HA������c��H+��+c��HA��-c��OH-��=c��Na+��= ��0.1mol/L=0.05mol/L��
��0.1mol/L=0.05mol/L��
�ʴ�Ϊ��0.05��
��3��pH=2��ϡ������Һ��C��H+��=10-2mol/L��pH=11��NaOH��Һ��C��H+��=10-11mol/L���������Ϻ���Һ�����ԣ���10-2mol/L=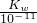 mol/L������KW=1.0��10-13��
mol/L������KW=1.0��10-13��
�ʴ�Ϊ��1.0��10-13��
�ٽ�100mL 0.1mol?L-1��ϡH2SO4��Һ��100mL 0.4mol?L-1��NaOH��Һ��Ϻ��������ƹ�������Ӧ��C��OH-��=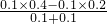 =0.1mol/L����C��H+��=
=0.1mol/L����C��H+��=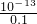 mol/L=10-12 mol/L���ʷ�Ӧ����Һ��pH=-lg10-12=12��
mol/L=10-12 mol/L���ʷ�Ӧ����Һ��pH=-lg10-12=12��
�ʴ�Ϊ��12��
��1�����ϡ�����10�����NaOH��Һ��Ϻ���Һ�����ԣ�˵�������ӵ����ʵ����������������ӵ����ʵ�����
��pHa=a��pHb=b����1��10-a=10��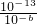 ����a+b=12����pHa+pHb=12��
����a+b=12����pHa+pHb=12��
�ʴ�Ϊ��pHa+pHb=12��
��������1�����ݲ����Ƿ�淶����ɵ���������
��2���ٸ���c��OH-��/c��H+��=1��10-8�����ˮ�����ӻ�����������Һ��������Ũ�ȣ��ٸ���pH=-lgc��H+�����㣻
HA���������Ʒ�Ӧ����NaA��ˮ�����ݼ����ж�HA����ǿ������ʣ��ݴ���д���ӷ���ʽ��
�ڸ�����ͼ�����ʵ�������Դ�Сȷ����Һ�е����ʣ��Ӷ�ȷ����Һ������ԣ���ϵ���ƽ�⡢ˮ��ƽ�⡢����غ��ȷ����Һ�и�������Ũ�ȵ���Դ�С��
�۸��ݵ���غ������غ��жϣ�
��3����ˮ�����ӻ�ΪKw����ʾ������������Һ�����������ӵ�Ũ�ȣ��������Ӧ���������������������Ũ�ȵ�������������Һ������������Ũ�ȣ��ݴ˽��
�ٵ������Ϻ��������ƹ�������Һ�ʼ��ԣ��ȼ���ʣ�����������ӵ�Ũ�ȣ��ٸ���ˮ�����ӻ�������Һ��������Ũ�ȣ��ٸ���pH=-lgc��H+�����㣻
����Һ�����ԣ�˵�������ӵ����ʵ����������������ӵ����ʵ������ݴ˼��㣮
���������⿼����Һ���Ƽ��к͵ζ�����������ҺpH�ļ��㡢����Ũ�ȱȽϵȣ���Ŀ�ۺ��Խϴ��Ѷ��еȣ���3��ע�����ˮ�����ӻ���Ϊ�״��㣮
B�����ʵ��������60mL ��ϡ�������Һ������ʱӦѡ��100mL����ƿ������ƿ�Ĺ����50mL��100mL������ƿ��Ӧѡ���Դ��ڻ����������Һ���������ƿ����B��ȷ��
C������ƿ�к�����������ˮ����Ӱ�����ʵ����ʵ�������Һ����������Բ��ᵼ���������Һ��Ũ��ƫС����C����
D����ʽ�ζ���������ˮϴ�Ӻ�װ���Ũ�ȵ�ϡ���ᣬ����ϡ�����Ũ��ƫС������ϡ��������ƫ�����Բ�õ�NaOH��Һ��Ũ�Ƚ�ƫ��D��ȷ��
E��������Һʱ���������һ�ζ���ʱ���Ӷ�����������Һ�����ƫСŨ��ƫ������������ƫС���ʵ���ʵ����ƫС����E����
F���к͵ζ�ʱ���������һ�ζ���ʱ���Ӷ�������������������ƫС���ʵ���ʵ����ƫС����F����
��ѡABD��
��2����c��OH-��/c��H+��=1��10-8��c��OH-����c��H+��=1.0��10-14����c��H+��=10-3mol?L-1��������Һ��pH=3��������Ũ��С�����Ũ�ȣ����Ը��������ᣬ���ᣨHA����NaOH��Һ��Ӧ�����ӷ���ʽΪ��HA+OH-�TA-+H2O��
�ʴ�Ϊ��3��HA+OH-�TA-+H2O��
��pH=11��NaOH��Һ��c��OH-��=10-3mol?L-1��HA�����ᣬ���Ũ��ԶԶ����������Ũ�ȣ�����pH=3��HA��pH=11��NaOH��Һ�������Ϻ���Һ�е�����������Σ���Һ�����ԣ�������Һ��������Ũ�ȴ�������������Ũ�ȣ��������Ũ�ȴ���������Ũ�ȣ���Һ��4���������ʵ���Ũ�ȴ�С��ϵ��c��A-����c��Na+����c��H+����c��OH-����
�ʴ�Ϊ��c��A-����c��Na+����c��H+����c��OH-����
��0.2mol?L-1HA��Һ��0.1mol?L-1NaOH��Һ�������Ϻ�������Һ�У��ɵ���غ��У�c��Na+��+c��H+��=c��A-��+c��OH-�����������غ��У�2c��Na+��=c��A-��+c��HA������c��H+��+c��HA��-c��OH-��=c��Na+��=
 ��0.1mol/L=0.05mol/L��
��0.1mol/L=0.05mol/L���ʴ�Ϊ��0.05��
��3��pH=2��ϡ������Һ��C��H+��=10-2mol/L��pH=11��NaOH��Һ��C��H+��=10-11mol/L���������Ϻ���Һ�����ԣ���10-2mol/L=
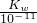 mol/L������KW=1.0��10-13��
mol/L������KW=1.0��10-13���ʴ�Ϊ��1.0��10-13��
�ٽ�100mL 0.1mol?L-1��ϡH2SO4��Һ��100mL 0.4mol?L-1��NaOH��Һ��Ϻ��������ƹ�������Ӧ��C��OH-��=
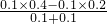 =0.1mol/L����C��H+��=
=0.1mol/L����C��H+��=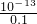 mol/L=10-12 mol/L���ʷ�Ӧ����Һ��pH=-lg10-12=12��
mol/L=10-12 mol/L���ʷ�Ӧ����Һ��pH=-lg10-12=12���ʴ�Ϊ��12��
��1�����ϡ�����10�����NaOH��Һ��Ϻ���Һ�����ԣ�˵�������ӵ����ʵ����������������ӵ����ʵ�����
��pHa=a��pHb=b����1��10-a=10��
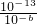 ����a+b=12����pHa+pHb=12��
����a+b=12����pHa+pHb=12���ʴ�Ϊ��pHa+pHb=12��
��������1�����ݲ����Ƿ�淶����ɵ���������
��2���ٸ���c��OH-��/c��H+��=1��10-8�����ˮ�����ӻ�����������Һ��������Ũ�ȣ��ٸ���pH=-lgc��H+�����㣻
HA���������Ʒ�Ӧ����NaA��ˮ�����ݼ����ж�HA����ǿ������ʣ��ݴ���д���ӷ���ʽ��
�ڸ�����ͼ�����ʵ�������Դ�Сȷ����Һ�е����ʣ��Ӷ�ȷ����Һ������ԣ���ϵ���ƽ�⡢ˮ��ƽ�⡢����غ��ȷ����Һ�и�������Ũ�ȵ���Դ�С��
�۸��ݵ���غ������غ��жϣ�
��3����ˮ�����ӻ�ΪKw����ʾ������������Һ�����������ӵ�Ũ�ȣ��������Ӧ���������������������Ũ�ȵ�������������Һ������������Ũ�ȣ��ݴ˽��
�ٵ������Ϻ��������ƹ�������Һ�ʼ��ԣ��ȼ���ʣ�����������ӵ�Ũ�ȣ��ٸ���ˮ�����ӻ�������Һ��������Ũ�ȣ��ٸ���pH=-lgc��H+�����㣻
����Һ�����ԣ�˵�������ӵ����ʵ����������������ӵ����ʵ������ݴ˼��㣮
���������⿼����Һ���Ƽ��к͵ζ�����������ҺpH�ļ��㡢����Ũ�ȱȽϵȣ���Ŀ�ۺ��Խϴ��Ѷ��еȣ���3��ע�����ˮ�����ӻ���Ϊ�״��㣮

��ϰ��ϵ�д�
 �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д� ������״Ԫ��ҵϵ�д�
������״Ԫ��ҵϵ�д� ��ʱ�ƿ�������ϰϵ�д�
��ʱ�ƿ�������ϰϵ�д�
�����Ŀ