��Ŀ����
��12�֣���1��ij�о���ѧϰС����ʵ����������1 mol/L��ϡ�������Һ��Ȼ������ζ�ijδ֪Ũ�ȵ�NaOH��Һ�������й�˵������ȷ����______________��
A��ʵ�������õ��ĵζ��ܡ�����ƿ����ʹ��ǰ����Ҫ��©��
B�����ʵ��������60 mL ��ϡ�������Һ������ʱӦѡ��100 mL����ƿ��
C������ƿ�к�����������ˮ���ᵼ���������Һ��Ũ��ƫС��
D����ʽ�ζ���������ˮϴ�Ӻ�װ���Ũ�ȵ�ϡ���ᣬ���õ�NaOH��Һ��Ũ�Ƚ�ƫ��
E��������Һʱ���������һ�ζ���ʱ���Ӷ������������ʵ����ƫ��
F���к͵ζ�ʱ���������һ�ζ���ʱ���Ӷ������������ʵ����ƫ��
��2�������£���֪0.1 mol��L��1һԪ��HA��Һ��c(OH��) / c(H+)��1��10��8��
�������£�0.1 mol��L��1 HA��Һ��pH= ��д�����ᣨHA����NaOH��Һ��Ӧ�����ӷ���ʽ�� ��
��pH��3��HA��pH��11��NaOH��Һ�������Ϻ���Һ��4���������ʵ���Ũ�ȴ�С��ϵ�ǣ� ��
��0.2 mol��L��1HA��Һ��0.1mol��L��1NaOH��Һ�������Ϻ�������Һ�У�
c(H+)��c(HA)��c(OH��)�� mol��L��1������Һ����仯���Բ��ƣ�
��3��t��ʱ����pH=2��ϡ�����pH=11��NaOH��Һ�������Ϻ���Һ�����ԣ�����¶���ˮ�����ӻ�����K w= ��
�ٸ��¶��£�t�棩����100 mL 0.1 mol��L-1��ϡH2SO4��Һ��100 mL 0.4 mol��L-1��NaOH��Һ��Ϻ���Һ����仯���Բ��ƣ�����Һ��pH= ��
�ڸ��¶��£�t�棩��1�����ϡ�����10�����NaOH��Һ��Ϻ���Һ�����ԣ���ϡ�����pH��pHa����NaOH��Һ��pH��pHb���Ĺ�ϵ�ǣ� ��
��12�֣�(1)ABD (2��)
(2) ��3
HA+OH ===A
===A +H2O ��c(A
+H2O ��c(A )>c(Na+)>c(H+)>c(OH
)>c(Na+)>c(H+)>c(OH )
��0.05 ��1��
)
��0.05 ��1��
(3)10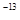 (2��) ��12
(2��)
��pHa+pHb=12(2��)
(2��) ��12
(2��)
��pHa+pHb=12(2��)
��������
�����������1������ƿ�к�����������ˮ������Ӱ�����ʺ��ܼ������������Բ�Ӱ��ʵ������C����ȷ��������Һʱ���������һ�ζ���ʱ���Ӷ�������Ũ��������ƫС���������ʵ����ƫ�ͣ�D����ȷ���к͵ζ�ʱ���������һ�ζ���ʱ���Ӷ���������������������С���������ʵ����ƫС��F����ȷ������ѡ�����ȷ�ģ���ѡABD��
(2) �ٸ���ˮ�����ӻ�������֪�����c(OH��) / c(H+)��1��10��8������Һ��OH������Ũ����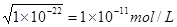 ����������Ũ����0.001mol/L������pH��3����˵��HA�����ᣬ���Ժ��������Ʒ�Ӧ�����ӷ���ʽ��HA+OH
����������Ũ����0.001mol/L������pH��3����˵��HA�����ᣬ���Ժ��������Ʒ�Ӧ�����ӷ���ʽ��HA+OH ===A
===A +H2O��
+H2O��
������HA�����ᣬ����pH��3��HA��Һ��Ũ�ȴ���0.001mol/L�����ں��������Ƶķ�Ӧ��HA�ǹ����ģ���˷�Ӧ����Һ�����ԣ�������Ũ�ȴ�С˳����c(A )>c(Na+)>c(H+)>c(OH
)>c(Na+)>c(H+)>c(OH )
��
)
��
��0.2 mol��L��1HA��Һ��0.1mol��L��1NaOH��Һ�������Ϻ����ǹ����ģ����Ը��ݵ���غ㶨�ɺ������غ��֪��c(Na+)��c(H+)��c(OH )��c(A
)��c(A )��2c(Na+)��c(HA)��c(A
)��2c(Na+)��c(HA)��c(A )����c(H+)��c(HA)��c(OH��)��c(Na+)��0.05mol/L��
)����c(H+)��c(HA)��c(OH��)��c(Na+)��0.05mol/L��
��3��t��ʱ����pH=2��ϡ�����pH=11��NaOH��Һ�������Ϻ���Һ�����ԣ���0.01��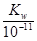 ����ø��¶���ˮ�����ӻ�����K w=10
����ø��¶���ˮ�����ӻ�����K w=10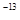 ��
��
��������������Ƶ����ʵ�����0.01mol��0.04mol�������������ǹ����ģ�������Һ��OH��Ũ���ǣ�0.04mol��0.02mol����0.2L��0.1mol/L������Һ��������Ũ����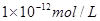 ����pH��12��
����pH��12��
��1�����ϡ�����10�����NaOH��Һ��Ϻ���Һ�����ԣ��� �����pHa+pHb=12��
�����pHa+pHb=12��
���㣺�����к͵ζ���ʵ���������������Һ������Ũ�ȴ�С�Ƚϼ�pH���йؼ����
�������ڱȽ���Һ������Ũ�ȴ�Сʱ��Ӧ�ó�����úõ���غ㶨�ɡ������غ��Լ������غ�ȹ�ϵʽ�����������ˮ�����ӻ����������йؼ���ʱ������ע��ˮ�����ӻ��������¶��й�ϵ�����ڵ��������ȵģ�����ˮ�����ӻ��������¶ȵ����߶�����ֻ���ڳ��³�ѹ��pH��7����Һ���������Եġ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�