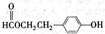��Ŀ����
��14�֣�
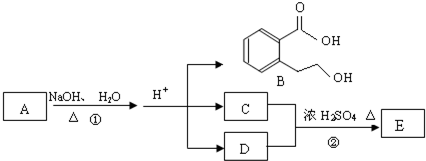
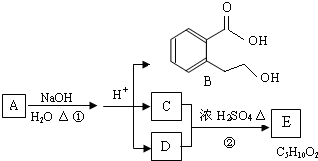
A��B��C��D��E��Ϊ��ѧ��ѧ�ij������ʻ������֮��ķ�Ӧ��ϵ��ͼ��ʾ��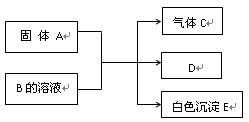
��1����A�Ƕ�������ԭ�Ӱ뾶����Ԫ�ع��ɵĵ��ʣ�E�ȿ����������ֿ�����NaOH��Һ��E����NaOH��Һ�����ӷ���ʽ�� ����ҵ��ұ��A�Ļ�ѧ��Ӧ����ʽ�� ��
��2����C�ǼȺ��м��Լ��ֺ��зǼ��Լ�����ԭ�ӷ��ӣ���ʵ������ȡC�Ļ�ѧ����ʽ�� ��1 mol C��ȫȼ������Һ̬ˮʱ����1300 kJ��mol-1����C��ȫȼ�յ��Ȼ� ѧ����ʽ�� ��A����B����Һ��Ӧʱֻ��������C��̼��Ƴ�����ˮ����B�Ļ�ѧʽ�� ��
ѧ����ʽ�� ��A����B����Һ��Ӧʱֻ��������C��̼��Ƴ�����ˮ����B�Ļ�ѧʽ�� ��
��1��Al(OH)3 + OH- = AlO2- + 2 H2O��3�֣���2 NaCl�����ڣ�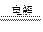 2 Na + Cl2����3�֣���
2 Na + Cl2����3�֣���
��2��CaC 2 + 2 H2O �� C2H2��+ Ca(OH)2��3�֣���
2 + 2 H2O �� C2H2��+ Ca(OH)2��3�֣���
2 C2H2(g) +5 O2(g) =" 4" CO2(g) + 2 H2O(l) ��H=" -" 2600 kJ��mol-1��3�֣���Ca(HCO3)2��2�֣�
����

��ϰ��ϵ�д�
 ��У��������ĩ��̾�ϵ�д�
��У��������ĩ��̾�ϵ�д�
�����Ŀ