��Ŀ����
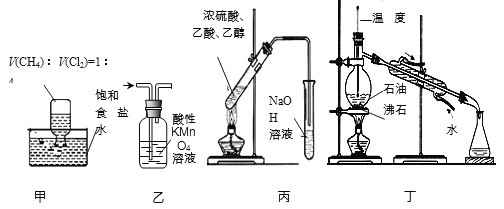
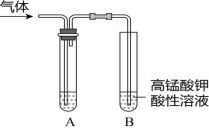
����Ŀ����ʵ�����Ƶ������г��������ʣ�Ӱ�������ʵļ��顣��ͼAΪ����װ�ã�BΪ���ʼ���װ�ã�������б���
��� | ���� | ��Ӧԭ�� | A���Լ� |
�� | ��ϩ | ��ˮ�Ҵ���Ũ���Ṳ�ȣ���Ӧ�Ļ�ѧ����ʽ��______ | ___ |
�� | ��ϩ | ��������NaOH���Ҵ���Һ���ȣ���Ӧ�Ļ�ѧ����ʽ��_____ | ___ |
�� | ��Ȳ | ���ʯ�еμӱ���ʳ��ˮ����Ӧ�Ļ�ѧ����ʽ��___ | ____ |
��Ϊ̽������������ˮ�������ijͬѧȡ��С��ͬ��3֧�Թܣ��ֱ����������Һ�����������ͬһˮԡ�м�����ͬʱ�䣬�۲쵽��������
�Թܱ�� | �� | �� | �� |
ʵ����� |
|
|
|
ʵ������ | ����䱡 | ������ʧ | ����������� |
��1���Թܢ��з�Ӧ�Ļ�ѧ����ʽ��__________��
��2������Թܢ�ʵ���������/span>__________��
��3��ʵ�������__________��
���𰸡�CH3CH2OH ![]() CH2=CH2����H2O NaOH��Һ CH3CH2Br��NaOH
CH2=CH2����H2O NaOH��Һ CH3CH2Br��NaOH![]() CH2=CH2����NaBr+ H2O ˮ CaC2��2H2O��CH��CH����Ca��OH��2 CuSO4��Һ CH3COOC2H5��NaOH
CH2=CH2����NaBr+ H2O ˮ CaC2��2H2O��CH��CH����Ca��OH��2 CuSO4��Һ CH3COOC2H5��NaOH ![]() CH3COONa��C2H5OH �Ա�ʵ�飬̽������������ˮ������ ���������ڲ�ͬ�����µ�ˮ��̶ȣ����ԣ����ԣ�����
CH3COONa��C2H5OH �Ա�ʵ�飬̽������������ˮ������ ���������ڲ�ͬ�����µ�ˮ��̶ȣ����ԣ����ԣ�����
��������
�����Ҵ�Ϊԭ������ϩ����Ҫ��Ũ��������������ˮ�����������¶���170�����ҡ�
��Ӧ�Ļ�ѧ����ʽΪCH3CH2OH ![]() CH2=CH2����H2O
CH2=CH2����H2O
����CH3CH2OH ![]() CH2=CH2����H2O��
CH2=CH2����H2O��
��Ϊ��ϩ�п��ܻ���Ũ���Ὣ�Ҵ�̼�������ɵ�SO2������Ӧʹ��NaOH��Һ���ӡ�
��Ϊ��NaOH��Һ��
������������NaOH���Ҵ���Һ��������ϩ��������Ӧ�Ļ�ѧ����ʽΪ
CH3CH2Br��NaOH![]() CH2=CH2����NaBr+ H2O��
CH2=CH2����NaBr+ H2O��
����CH3CH2Br��NaOH![]() CH2=CH2����NaBr+ H2O��
CH2=CH2����NaBr+ H2O��
��Ȼ����������ˮ��ĸ���Ӧ�������ﲻ������ϩ�����У���������ֻ��Ϊ�Ҵ������ܼ��ӷ����£�����ˮ�����Ҵ�����Ϊ��ˮ��
�����ʯ�еμӱ���ʳ��ˮ��������Ӧ�Ļ�ѧ����ʽΪCaC2��2H2O��CH��CH����Ca(OH)2��
����CaC2��2H2O��CH��CH����Ca(OH)2
��Ϊ��ʯ�г�����CaS���ʣ��������ɵ���Ȳ�����г�����H2S���壬��ʹ������ͭ��Һ��������Ϊ��CuSO4��Һ��
��1���Թܢ���Ϊ���������������������µ�ˮ�ⷴӦ����Ӧ��ȫ����Ӧ�Ļ�ѧ����ʽ��CH3COOC2H5��NaOH ![]() CH3COONa��C2H5OH��
CH3COONa��C2H5OH��
����CH3COOC2H5��NaOH ![]() CH3COONa��C2H5OH��
CH3COONa��C2H5OH��
��2���Թܢ���δ�Ӵ���������������ˮ�ⷴӦ������Ϊ�Ա�ʵ���õġ�
��Ϊ���Ա�ʵ�飬̽������������ˮ��������
��3�������������֪�����������ڼ�����������ȫˮ�⣻��ʵ������ķ�����֪�������������²���ˮ�⣻��ˮ��Һ�У���������ˮ��̶Ⱥ�С�����ԣ�ʵ����������������ڲ�ͬ�����µ�ˮ��̶ȣ����ԣ����ԣ�����
��Ϊ�����������ڲ�ͬ�����µ�ˮ��̶ȣ����ԣ����ԣ����ԡ�

����Ŀ����֪T��W��X��Y��Z��Ԫ�����ڱ�ǰ�������еij���Ԫ�أ�ԭ�������������������Ϣ�����
Ԫ�� | �����Ϣ |
T | TԪ�ؿ��γ���Ȼ��Ӳ�����ĵ��� |
W | W��Tͬ���ڣ�������һ��δ�ɶԵ��� |
X | Xԭ�ӵĵ�һ�����������ĵ����ֱܷ��ǣ�I1=578kJ��mol-1��I2=1817kJ��mol-1��I3=2745kJ��mol-1��I4=11575kJ��mol-1 |
Y | ���³�ѹ�£�Y�����ǹ��壬�����������γ��������Ҫ���� |
Z | Z��һ��ͬλ�ص�������Ϊ63��������Ϊ34 |
��1��TY2��һ�ֳ��õ��ܼ�����__(�������Է����������Ǽ��Է�����)�������д��� ___��![]() ����W������⻯������Һ����������___��
����W������⻯������Һ����������___��
��2����25�桢101kPa�£���֪13.5g��X���嵥����O2����ȫȼ�պ�ָ���ԭ״̬������419kJ���÷�Ӧ���Ȼ�ѧ����ʽΪ__��
��3����̬Yԭ���У�����ռ�ݵ�����ܲ����Ϊ__�����ܲ���е�ԭ�ӹ����Ϊ___��������Ϊ___��Y������WԪ�صĵ縺���ɴ�С��˳��Ϊ___(��Ԫ�ط�������)��
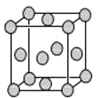
��4����֪Z�ľ����ṹ��ͼ��ʾ����֪Z���ܶ�Ϊ9.00g��cm-3�����߳�Ϊ___��
ZYO4�������Һ������YO42-�Ŀռ乹����__������Yԭ�ӵ��ӻ����������___��Z�ĵ���������������е������Լ�������Ӧ�����ɳ����Z+HCl+O2=ZCl+HO2��HO2(������![]() ������һ���������Ҳ��һ�����ɻ������м��ߵĻ��ԡ�����˵�����ʾ��ȷ����__��
������һ���������Ҳ��һ�����ɻ������м��ߵĻ��ԡ�����˵�����ʾ��ȷ����__��
A.O2�������� B.HO2����������
C.HO2�ڼ������ȶ����� D.1molZ�μӷ�Ӧ��1mol���ӷ���ת��