��Ŀ����
6������һ������ˮ��Һ��ֻ���ܺ������������е������֣�K+��NH${\;}_{4}^{+}$��Cl-��Mg2+��Ba2+��CO${\;}_{3}^{2-}$��SO${\;}_{4}^{2-}$����ȡ������Һ��100mL��������ʵ�飺��1����һ�ݼ���AgNO2��Һ�г���������
��2���ڶ��ݼ�����NaOH��Һ���Ⱥ��ռ�������0.04mol��
��3�������ݼ�����BaCl2��Һ�ø������6.63g������������ϴ�ӡ������������Ϊ4.66g������֪NH${\;}_{4}^{+}$��OH-�ķ�ӦΪNH${\;}_{4}^{+}$+OH-$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+H2O����������ʵ�飬�����Ʋ��в���ȷ���ǣ�������
| A�� | 100mL��Һ��һ��ֻ��0.02molK+ | B�� | ��Һ��CO${\;}_{3}^{2-}$Ũ��Ϊ0.1mol/L | ||
| C�� | ����ȷ��Cl-�Ƿ���� | D�� | Ba2+��Mg2+һ�������� |
���� ��1������AgNO3��Һ�г���������˵����Һ�п��ܴ���Cl-��CO32-��SO42-��
��2��0.04molΪ��������Һ��һ������NH4+���������ʵ���Ϊ0.04mol��
��3��4.66gΪ���ᱵ��6.63gΪ���ᱵ��̼�ᱵ���ٸ��ݵ���غ㣬�ó�һ�����ڼ����ӣ�
��� �⣺��1����AgNO3��Һ�г���������������Cl-��CO32-��SO42-����2��������NaOH��Һ���Ȳ������壬�����ǰ�������һ���������0.04mol��
��3�������������4.66gΪ���ᱵ�����ʵ����ǣ�$\frac{4.66g}{233g/mol}$=0.02mol��6.63g���������ᱵ��̼�ᱵ��̼�ᱵ����Ϊ6.63g-4.66g=1.97g��̼�ᱵ�����ʵ���Ϊ��$\frac{1.97g}{197g/mol}$=0.01mol����һ������CO32-��SO42-�����һ��û�� Mg2+��Ba2+��
���������غ��֪��n��CO32-��=n��BaCO3��=0.01 mol��n��SO42-��=n��BaSO4��=0.02mol��
�ٸ��ݵ���غ㣬�����Ϊ��n��+��=n��NH4+��=0.04mol��c��-��=2c��CO32-��+2c��SO42-��=0.06mol����һ����K+������0.02mol��
�ۺ����Ͽ��Եó���һ�����ڵ�������NH4+��K+��CO32-��SO42-��һ��û�е�����Mg2+��Ba2+�����ܴ���Cl-��
A�����������ƶϣ�һ�����ڼ����ӣ������ʵ�����0.02mol����A����
B��̼������ӵ����ʵ�����0.01mol��Ũ��Ϊ��$\frac{0.01mol}{0.1L}$=0.1mol/L����B��ȷ��
C������ȷ���Ƿ��������ӣ���C��ȷ��
D����Һ��һ��û�е�����Mg2+��Ba2+����D��ȷ��
��ѡA��
���� ���⿼�鳣���ӵļ��鷽������Ŀ�Ѷ��еȣ���ȷ�������ӵ����ʼ����鷽��Ϊ���ؼ������õ���غ��ƶϼ����ӵĴ������Ϊ�״��㣬����������ѧ���ķ���������������������

 ��һ������Ԫͬ�����ؾ�ϵ�д�
��һ������Ԫͬ�����ؾ�ϵ�д�| A�� | H2O �� g ���TH2 �� g ��+$\frac{1}{2}$O2 �� g ����H=+242 kJ/mol | |
| B�� | 2H2 �� g ��+O2�� g ���T2H2O �� l ����H=-484 kJ/mol | |
| C�� | H2 �� g ��+$\frac{1}{2}$O2 �� g ���TH2O ��g ����H=+242 kJ/mol | |
| D�� | 2H2 �� g ��+O2 �� g ���T2H2O �� g ����H=+484 kJ/mol |
| A�� | �����¶ȣ�KW����pH������Ϊ���� | |
| B�� | ��ˮ�м����������ᣬc��H+������ | |
| C�� | ��ˮ�м��백ˮ��ƽ�����淴Ӧ�����ƶ���c��OH-������ | |
| D�� | һ���¶��£���ˮ�м�����������CH3COONa��ƽ��������Ӧ�����ƶ���Kw���� |
| A�� | H2O+H2O?H3O++OH- | B�� | HCO3-+OH-?H2O+CO32- | ||
| C�� | CO2+H2O?H2CO3 | D�� | CO32-+H2O?HCO3-+OH- |
| A�� | FeCl3 | B�� | NH4Cl | C�� | NaAlO2 | D�� | Cu��NO3��2 |
| A�� | ���ʯ��ʯī��Ϊ̼Ԫ����ɵĵ��� | |
| B�� | CO2��������� | |
| C�� | ������CuSO4•5H2O���ɱ����ڻ��������ʯ���ڻ���� | |
| D�� | ���һԪ���� |
| A�� | 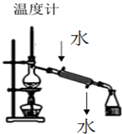 װ�����ڷ������ַе����ϴ��Һ�廥�ܻ���� | |
| B�� |  װ�ó����������ƹ��� | |
| C�� |  װ������һ�����ʵ���Ũ�ȵ�ϡ���� | |
| D�� |  װ�ü������ƿ�Ƿ�©ˮ |