��Ŀ����
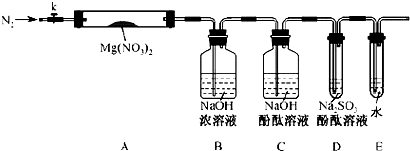
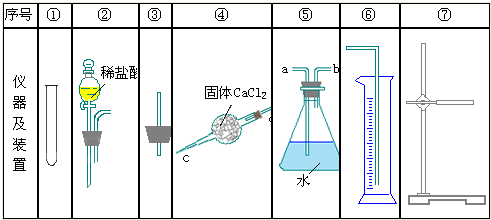
������ͼ�ṩ������װ�ã�����ᴿ���ⶨNa2CO3��Ʒ���ȣ�����ֻ��NaHCO3���ʣ���ʵ�飮
��ش��������⣺
ʵ��һ���ⶨNa2CO3��Ʒ�е�NaHCO3�ֽ�ų��Ķ�����̼�������
��1������װ�õ���ȷ˳���ǣ�______��______��______��______����д��ţ�
��2�����ʵ��һ��ȱ�������ǣ�______��
��3������Ӧ��װ�âݵ�______�˽��룮��д��ĸ��
ʵ������ⶨ��ʵ��һ��Ĺ������������ᷴӦ�ų�������̼�������
��4������װ�õ���ȷ˳���ǣ�______��______��______��______��д��ţ�
��5����ʵ��һ��ò����Ķ�����̼Ϊamol����ʵ�����ò����Ķ�����̼Ϊbmol�������Ʒ��̼���ƴ��ȵ�ʵ��ֵΪ��______��������ֵ�Ƚϣ�ʵ����______���ƫ��ƫС����ȣ�����Ҫʹʵ��ֵ���ӽ�������ֵ����ʵ��Ӧ�����Ľ���______�������ּ�Ҫ˵����

��ش��������⣺
ʵ��һ���ⶨNa2CO3��Ʒ�е�NaHCO3�ֽ�ų��Ķ�����̼�������
��1������װ�õ���ȷ˳���ǣ�______��______��______��______����д��ţ�
��2�����ʵ��һ��ȱ�������ǣ�______��
��3������Ӧ��װ�âݵ�______�˽��룮��д��ĸ��
ʵ������ⶨ��ʵ��һ��Ĺ������������ᷴӦ�ų�������̼�������
��4������װ�õ���ȷ˳���ǣ�______��______��______��______��д��ţ�
��5����ʵ��һ��ò����Ķ�����̼Ϊamol����ʵ�����ò����Ķ�����̼Ϊbmol�������Ʒ��̼���ƴ��ȵ�ʵ��ֵΪ��______��������ֵ�Ƚϣ�ʵ����______���ƫ��ƫС����ȣ�����Ҫʹʵ��ֵ���ӽ�������ֵ����ʵ��Ӧ�����Ľ���______�������ּ�Ҫ˵����
��1���ⶨNa2CO3��Ʒ�е�NaHCO3�ֽ�ų��Ķ�����̼��������ɽ�������������Թ��м��ȣ����ɵĶ�����̼��������ˮ�����������������˳��Ϊ�٢ۢݢޣ�
�ʴ�Ϊ���٢ۢݢޣ�
��2����ӦӦ�ڼ��������½��У�ʵ����ȱ�پƾ��ƣ��ʴ�Ϊ���ƾ��ƣ�
��3������ˮ��������������ʱ������Ӧ�̽��������ʴ�Ϊ��b��
��4���ⶨ��ʵ��һ��Ĺ������������ᷴӦ�ų�������̼�������Ӧ����������Թ��У�����������Һ©���У�Ȼ������ˮ��������������������˳��Ϊ�٢ڢݢޣ�
�ʴ�Ϊ���٢ڢݢޣ�
��5����ʵ��һ��ò����Ķ�����̼Ϊa mol����ʵ�����ò����Ķ�����̼Ϊb mol��
��Ӧ����ط���ʽΪ��2NaHCO3
Na2CO3+CO2��+H2O��Na2CO3+2HCl�T2NaCl+CO2��+H2O��
���������xmolNaHCO3��ymolNa2CO3��
����
��֮��y=b-a��
����Ʒ��̼���Ƶ�����Ϊ106��b-a��g��NaHCO3������Ϊ84��2a=168ag��
����Ʒ��̼���ƴ��ȵ�ʵ��ֵΪ
��100%��
�������̼����ˮ�������ռ�������ƫС����������ֵ�Ƚϣ�ʵ����ƫС��Ӧ��װ�âݵ�ˮ���ɱ���NaHCO3��Һ��
�ʴ�Ϊ��
��100%��ƫС����װ�âݵ�ˮ���ɱ���NaHCO3��Һ��
�ʴ�Ϊ���٢ۢݢޣ�
��2����ӦӦ�ڼ��������½��У�ʵ����ȱ�پƾ��ƣ��ʴ�Ϊ���ƾ��ƣ�
��3������ˮ��������������ʱ������Ӧ�̽��������ʴ�Ϊ��b��
��4���ⶨ��ʵ��һ��Ĺ������������ᷴӦ�ų�������̼�������Ӧ����������Թ��У�����������Һ©���У�Ȼ������ˮ��������������������˳��Ϊ�٢ڢݢޣ�
�ʴ�Ϊ���٢ڢݢޣ�
��5����ʵ��һ��ò����Ķ�����̼Ϊa mol����ʵ�����ò����Ķ�����̼Ϊb mol��
��Ӧ����ط���ʽΪ��2NaHCO3
| ||
���������xmolNaHCO3��ymolNa2CO3��
����
|
��֮��y=b-a��
����Ʒ��̼���Ƶ�����Ϊ106��b-a��g��NaHCO3������Ϊ84��2a=168ag��
����Ʒ��̼���ƴ��ȵ�ʵ��ֵΪ
| 106(b-a) |
| 168a+106(b-a) |
�������̼����ˮ�������ռ�������ƫС����������ֵ�Ƚϣ�ʵ����ƫС��Ӧ��װ�âݵ�ˮ���ɱ���NaHCO3��Һ��
�ʴ�Ϊ��
| 106(b-a) |
| 168a+106(b-a) |

��ϰ��ϵ�д�
�����Ŀ