��Ŀ����
��NAΪ�����ӵ���������ֵ������˵������ȷ����
| A����⾫����ͭʱ��ÿת��NA�����ӣ������ܽ�32gͭ |
| B��1L0��1mo1��L��1�İ�ˮ�к�OH��������Ϊ0��lNA�� |
| C�����³�ѹ�£�a mo1O2��2a mo1NO��ϣ����µõ������������ԭ����С��4aNA |
D���ڷ�Ӧ5NH4NO3 2HNO3+4N2��+9H2O�У�ÿ����4molN2��ת�Ƶ�����Ϊ15NA 2HNO3+4N2��+9H2O�У�ÿ����4molN2��ת�Ƶ�����Ϊ15NA |
D
�������������A����⾫����ͭʱ��ÿת��NA�����ӣ���������ͭ�ŵ磬�������������ŵ磬�ܽ�ҪС��32gͭ�� B��һˮ�ϰ�����������ʣ�1L0��1mo1��L��1�İ�ˮ�к�OH��������ҪԶԶС��Ϊ0��lNA����C�����³�ѹ�£�a mo1O2��2a mo1NO��ϣ���4molO����ԭ��������4aNA��
���㣺���鰢��٤��������������������֪ʶ��

��ϰ��ϵ�д�
�����Ŀ
�����йػ�ѧ�����ʾ��ȷ����
A�����Ȼ�̼���ӱ���ģ�ͣ� |
| B��������ĽṹʽΪ H-Cl-O |
C��COS�ĵ���ʽ��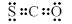 |
D��O2�����ӽṹʾ��ͼ�� |
��N0��ʾ�����ӵ�������ֵ������˵����ȷ����
| A����״���£�0.1 mol Cl2������������Һ��ȫ���գ�ת�Ƶĵ�����ĿΪ0.2N0 |
| B�����³�ѹ�£�16 g CH���к��е�ԭ������Ϊ5N0 |
| C����״���£�11.2 LCH3OH�к��е���ԭ����ĿΪ2N0 |
| D�����³�ѹ�£�2.24 L CO��CO2��������к��е�̼ԭ����ĿΪ0.1N0 |
�����йػ�ѧ�����ʾ��ȷ����
A��Beԭ�ӵĽṹʾ��ͼ��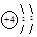 |
B������Ľṹ��ʽ��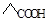 |
C��������Ϊ16����ԭ�ӣ�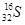 |
D��NH4Cl�ĵ���ʽ��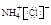 |
��NAΪ�����ӵ�������ֵ������˵����ȷ���� �� ��
| A��1L0.1mol��L��1�İ�ˮ�к��е�NH3������Ϊ0.1NA |
| B����״���£�2. 24L��CCl4�к��е�C��Cl����Ϊ0.4NA |
| C�����³�ѹ�£�3.0g�����Ǻͱ�����Ļ�����к��е�ԭ������Ϊ0.4NA |
| D����״���£�Na2O2������CO2��Ӧ����2.24L O2��ת�Ƶ�����Ϊ0.4NA |
��amolС�մ��bmol������������ij����ɱ���ܱ������г�ּ��ȣ���Ӧ���������ڵ�����Ϊ1 mol������˵��һ����ȷ����
| A��b="2" |
| B����Ӧ��ת�Ƶĵ�����һ��Ϊ2NA |
| C��������һ��û�в����CO2��ˮ���� |
| D��a��b��1 |
��NAΪ�����ӵ�������ֵ������������ȷ����
| A����״���£�2.24 Lˮ��������������ΪNA |
| B��1 L 0.2 mol��L��1��������Һ�к��е�SO42����Ϊ0.2NA |
| C�����ͱ���������1 mol����ȫȼ������O2�ķ�����Ϊ7.5NA |
| D����״���£�7.1 g����������ʯ�����ַ�Ӧת�Ƶ�����Ϊ0.2NA |
�谢���ӵ�������NA������ֵΪnA������˵����ȷ���ǣ����ԭ��������H��1��O��16��
| A��11.2LNH3����������Ϊ0.5nA |
| B��1molCl2������Fe��Ӧ��ת�Ƶĵ�����Ϊ3nA |
| C��100mL1mol/LFeCl3��Һ�к�Fe3+������Ŀ��0.1nA |
| D�������£�34gH2O2��H��O����ĿΪ2nA |
��NA��ʾ�����ӵ�������ֵ������������ȷ���ǣ� ��
| A�����³�ѹ�£�18gH218O���е�ԭ������Ϊ3NA |
| B����״���£�4.2gCH2=CH2��CH3CH=CH2�Ļ�������������е�̼ԭ����Ϊ0.3NA |
| C����0.1molCl2ͨ��1Lˮ�У�ת�Ƶĵ�����ĿΪ0.1NA |
| D��0.1mol��L-1Al2(SO4)3��Һ�к��е�Al3+����Ϊ0.2NA |