题目内容
(共13分)下表是元素周期表的一部分, 针对表中的①~⑨种元素,填写下列空白:
(1) 在这些元素中,化学性质最不活泼的是: (填具体元素符号,下同)。
(2) 在最高价氧化物的水化物中,酸性最强的化合物的分子式是_______,碱性最强的化合物的电子式是:_____________。
(3) 最高价氧化物是两性氧化物的元素是_________;写出它的氧化物与氢氧化钠反应的离子方程式_____________________________________________。
(4) 用电子式表示元素④与⑥的化合物的形成过程: ,该化合物属于 (填 “共价”或“离子”)化合物。
(5)表示①与⑦的化合物的电子式 ,该化合物是由
(填“极性”“非极性”)键形成的。
(6)写出元素⑦的单质与水反应的离子方程式
| 主族 周期 | ⅠA | ⅡA | ⅢA | ⅣA | ⅤA | ⅥA | ⅦA | 0族 |
| 2 | | | | ① | ② | ③ | | |
| 3 | ④ | | ⑤ | | | ⑥ | ⑦ | ⑧ |
| 4 | ⑨ | | | | | | | |
(2) 在最高价氧化物的水化物中,酸性最强的化合物的分子式是_______,碱性最强的化合物的电子式是:_____________。
(3) 最高价氧化物是两性氧化物的元素是_________;写出它的氧化物与氢氧化钠反应的离子方程式_____________________________________________。
(4) 用电子式表示元素④与⑥的化合物的形成过程: ,该化合物属于 (填 “共价”或“离子”)化合物。
(5)表示①与⑦的化合物的电子式 ,该化合物是由
(填“极性”“非极性”)键形成的。
(6)写出元素⑦的单质与水反应的离子方程式
(方程式、形成过程每空2分,其余每空1分)
(1)Ar; (2)HClO4;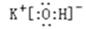 ; (3)Al; Al2O3+2OH-=2AlO2-+H2O
; (3)Al; Al2O3+2OH-=2AlO2-+H2O
(4)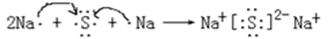 ; 离子
; 离子
(5)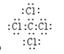 ; 极性共价键 (6)Cl2 +H2O =" H" ++ Cl- + HClO
; 极性共价键 (6)Cl2 +H2O =" H" ++ Cl- + HClO
(1)Ar; (2)HClO4;
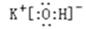 ; (3)Al; Al2O3+2OH-=2AlO2-+H2O
; (3)Al; Al2O3+2OH-=2AlO2-+H2O(4)
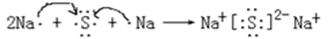 ; 离子
; 离子(5)
 ; 极性共价键 (6)Cl2 +H2O =" H" ++ Cl- + HClO
; 极性共价键 (6)Cl2 +H2O =" H" ++ Cl- + HClO考查元素周期表的结构及元素周期律的应用。根据元素在周期表中的位置可知,①~⑨种元素分别是C、N、O、Na、Al、S、Cl、Ar、K。
(1)稀有气体元素的最外层电子数已经达到平衡状态,所以化学性质最变化活泼。
(2)非金属性越强,最高价氧化物的水化物的酸性越强,因此是HClO4;同样金属性越强,最高价氧化物的水化物的碱性越强,所以是KOH,电子式是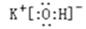 。
。
(3铝的氧化物氧化铝是两性氧化物,既能和酸赋,也能和碱反应。与氢氧化钠反应的方程式是Al2O3+2OH-=2AlO2-+H2O。
(4)钠和硫形成的化合物是硫化钠,属于含有离子键的离子化合物,其形成过程可表示为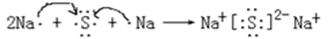 。
。
(5)C和Cl形成的四氯化碳,是含有极性键的非极性分子,其电子式是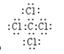 。
。
(6)氯气溶于水生成盐酸和次氯酸,方程式是Cl2 +H2O =" H" ++ Cl- + HClO。
(1)稀有气体元素的最外层电子数已经达到平衡状态,所以化学性质最变化活泼。
(2)非金属性越强,最高价氧化物的水化物的酸性越强,因此是HClO4;同样金属性越强,最高价氧化物的水化物的碱性越强,所以是KOH,电子式是
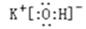 。
。(3铝的氧化物氧化铝是两性氧化物,既能和酸赋,也能和碱反应。与氢氧化钠反应的方程式是Al2O3+2OH-=2AlO2-+H2O。
(4)钠和硫形成的化合物是硫化钠,属于含有离子键的离子化合物,其形成过程可表示为
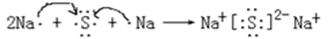 。
。(5)C和Cl形成的四氯化碳,是含有极性键的非极性分子,其电子式是
 。
。(6)氯气溶于水生成盐酸和次氯酸,方程式是Cl2 +H2O =" H" ++ Cl- + HClO。

练习册系列答案
相关题目

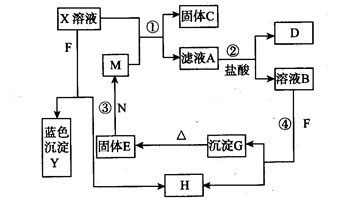
 溶液,生成白色沉淀,过滤后得滤液C;(3)将C分成两份,在一份中加入烧碱溶液,可得蓝色絮状沉淀D;(4)往另一份C中放入一枚新铁钉,有单质E析出。已知A、B、C、D、E相互关系如图所示。
溶液,生成白色沉淀,过滤后得滤液C;(3)将C分成两份,在一份中加入烧碱溶液,可得蓝色絮状沉淀D;(4)往另一份C中放入一枚新铁钉,有单质E析出。已知A、B、C、D、E相互关系如图所示。