��Ŀ����
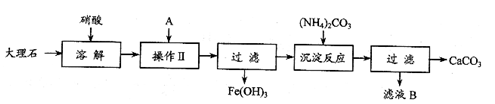
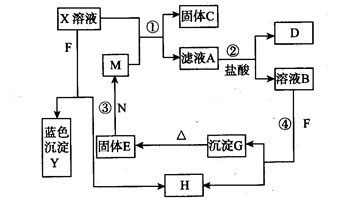
(13��)��֪XΪ��ѧ��ѧ��һ�ֳ������Σ�FΪ����ɫ���壻M��NΪ�����Ľ�����N������������ͻ���ϣ��������������ͻ���������������������HΪ���嵥�ʣ�DΪ��ɫ���壬D�ڿ����л���ֺ���ɫ�������ʵ�ת����ϵ����ͼ(���ַ�Ӧ������ȥ)��
��ش��������⣺
(1)E�Ļ�ѧʽΪ_______________(2��)��
(2)��M˿����ʢ��X��Һ���Թ��У���Ӧһ��ʱ����������_______________(2��)��
(3)�ڷ�Ӧ�٢ڢۢ��������û���Ӧ����__________(�����)(2��)��
(4)��Ӧ�ڵ����ӷ���ʽΪ______________________________________________(2��)��
(5)��ʯī���缫���50mL X��Һ���۲쵽A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɣ����һ��ʱ���ȡ��A�缫��ϴ�ӡ�����������缫����1.6g����ش��������⣺
��д�����ʱ��������Ӧʽ________________________________________(2��)��
�ڵ�����Һ��pHΪ_______________________________(3��)(������ǰ����Һ�������)��

��ش��������⣺
(1)E�Ļ�ѧʽΪ_______________(2��)��
(2)��M˿����ʢ��X��Һ���Թ��У���Ӧһ��ʱ����������_______________(2��)��
(3)�ڷ�Ӧ�٢ڢۢ��������û���Ӧ����__________(�����)(2��)��
(4)��Ӧ�ڵ����ӷ���ʽΪ______________________________________________(2��)��
(5)��ʯī���缫���50mL X��Һ���۲쵽A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɣ����һ��ʱ���ȡ��A�缫��ϴ�ӡ�����������缫����1.6g����ش��������⣺
��д�����ʱ��������Ӧʽ________________________________________(2��)��
�ڵ�����Һ��pHΪ_______________________________(3��)(������ǰ����Һ�������)��
(1)Fe2O3(2��) (2)��˿�ϸ��к�ɫ���ʣ���Һ��ɫ��dz(2��)
(3)(1)(3)(2��) (4)2Fe2++NO3-+4H+=3Fe3++NO��+2H2O(2��)
(5)��4OH--4e-=O2+2H2O(2��) ��0(3��)
(3)(1)(3)(2��) (4)2Fe2++NO3-+4H+=3Fe3++NO��+2H2O(2��)
(5)��4OH--4e-=O2+2H2O(2��) ��0(3��)
FΪ����ɫ���壬����Ϊ���Na2O2����������ҺB����ҺX��������ɫ����H������Y����ɫ�����ض�ΪCu(OH)2����G�����ж�FΪNa2O2��HΪ������XΪͭ�Σ�
�ɡ�N������������ͻ���ϣ��������������ͻ���������������������֪��N��Ϊ����������ˮ����������G������ˮ����������E��E������������һ��������M����֪���������ȷ�Ӧ������������E��Fe2O3�û���M�������ʣ���2Al��Fe2O3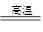 Al2O3��2Fe
Al2O3��2Fe
����ͭ��M�����û���C��ͭ����ҺA����������Һ���������������NO��DΪ��ɫ���壬D�ڿ����л���ֺ���ɫ��˵��Dһ��ΪNO���ڿ����б�����Ϊ����ɫ��NO2����˵��A�ض�Ϊ�����Σ���2Fe2++NO3-+4H+=3Fe3++NO��+2H2O���Ӷ���֪XΪ����ͭ��AΪ����������BΪ��������Һ��
(1)Fe2O3
(2)��������ͭ�����û���Ӧ����Fe��CuSO4=FeSO4��Cu������˿�ϸ��к�ɫ���ʣ���Һ��ɫ����ɫת����dz��ɫ
(3)��Ӧ�ܣ�2Na2O2��2H2O=4NaOH��O2�� FeCl3��3NaOH=Fe(OH)3����3NaCl���٢ڢۢ������û���Ӧ���Ǣ٢�
(4)2Fe2++NO3-+4H+=3Fe3++NO��+2H2O
(5)�ܵ�ⷴӦʽΪ��2Cu(NO3)2��2H2O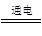 2Cu��O2����4HNO3
2Cu��O2����4HNO3
�����������������ɣ�4OH--4e-=O2��+2H2O
�����ܷ�Ӧʽ��֪��ͭΪ1.6g��ͬʱ�õ�n(HNO3)=0.05mol��c(HNO3)=0.05mol/0.05L=1mol/L
c(H+)=1mol/L��pH=0
�ɡ�N������������ͻ���ϣ��������������ͻ���������������������֪��N��Ϊ����������ˮ����������G������ˮ����������E��E������������һ��������M����֪���������ȷ�Ӧ������������E��Fe2O3�û���M�������ʣ���2Al��Fe2O3
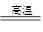 Al2O3��2Fe
Al2O3��2Fe����ͭ��M�����û���C��ͭ����ҺA����������Һ���������������NO��DΪ��ɫ���壬D�ڿ����л���ֺ���ɫ��˵��Dһ��ΪNO���ڿ����б�����Ϊ����ɫ��NO2����˵��A�ض�Ϊ�����Σ���2Fe2++NO3-+4H+=3Fe3++NO��+2H2O���Ӷ���֪XΪ����ͭ��AΪ����������BΪ��������Һ��
(1)Fe2O3
(2)��������ͭ�����û���Ӧ����Fe��CuSO4=FeSO4��Cu������˿�ϸ��к�ɫ���ʣ���Һ��ɫ����ɫת����dz��ɫ
(3)��Ӧ�ܣ�2Na2O2��2H2O=4NaOH��O2�� FeCl3��3NaOH=Fe(OH)3����3NaCl���٢ڢۢ������û���Ӧ���Ǣ٢�
(4)2Fe2++NO3-+4H+=3Fe3++NO��+2H2O
(5)�ܵ�ⷴӦʽΪ��2Cu(NO3)2��2H2O
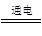 2Cu��O2����4HNO3
2Cu��O2����4HNO3�����������������ɣ�4OH--4e-=O2��+2H2O
�����ܷ�Ӧʽ��֪��ͭΪ1.6g��ͬʱ�õ�n(HNO3)=0.05mol��c(HNO3)=0.05mol/0.05L=1mol/L
c(H+)=1mol/L��pH=0

��ϰ��ϵ�д�
�����Ŀ

 ________��
________�� ɫ��˵����Һ�����ԣ���ԭ���Ƿ���ͬ�����ñ�Ҫ�����ּ��Խ��Ͳ�д�����ӷ���ʽ��
ɫ��˵����Һ�����ԣ���ԭ���Ƿ���ͬ�����ñ�Ҫ�����ּ��Խ��Ͳ�д�����ӷ���ʽ��