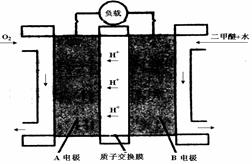
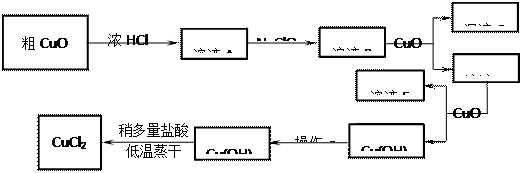
��Ŀ����
����ѧ����ѧ�뼼����������һ����Ҫ�Ļ���ԭ�ϣ����Ṥҵ�ġ����ϡ�����֮һ�Ƕ�β������������һ�ַ�����������Ȼ��ˮ����β������
��1�����Ṥҵ��������Ҫ�豸�� �� ��������������������װ�д����Ż��������� ��Ũ������������£�SO3���ղ�����ȡ ��ʽ��ԭ���������Ṥҵ��β����Ҫ�ɷ���SO2��O2��N2�ȡ�
��2����Ȼ��ˮ��Ҫ���� �����ӡ�
�����ӡ�
����Ȼ��ˮpHԼΪ8��ԭ���Ǻ�ˮ�� ����ˮ�����¡�
��β�������������ڣ��������´�����ˮ��O2�ܽ� ��
��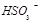 ��
��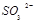 ����������д������һ����Ӧ�ķ���ʽ�� ��
����������д������һ����Ӧ�ķ���ʽ�� ��
����������ĺ�ˮ�м�����Ȼ��ˮ����Ŀ�����к͡�ϡ��������ˮ�����ɵ��ᣬ�����ŷų��ĺ�ˮ��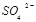 Ũ�����������������Ȼ��ˮ��ȣ�
Ũ�����������������Ȼ��ˮ��ȣ�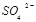 Ũ�� ������ţ���
Ũ�� ������ţ���
��1�����Ṥҵ��������Ҫ�豸�� �� ��������������������װ�д����Ż��������� ��Ũ������������£�SO3���ղ�����ȡ ��ʽ��ԭ���������Ṥҵ��β����Ҫ�ɷ���SO2��O2��N2�ȡ�
��2����Ȼ��ˮ��Ҫ����
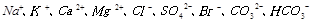 �����ӡ�
�����ӡ�����Ȼ��ˮpHԼΪ8��ԭ���Ǻ�ˮ�� ����ˮ�����¡�
��β�������������ڣ��������´�����ˮ��O2�ܽ�
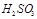 ��
��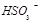 ��
��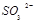 ����������д������һ����Ӧ�ķ���ʽ�� ��
����������д������һ����Ӧ�ķ���ʽ�� ������������ĺ�ˮ�м�����Ȼ��ˮ����Ŀ�����к͡�ϡ��������ˮ�����ɵ��ᣬ�����ŷų��ĺ�ˮ��
 Ũ�����������������Ȼ��ˮ��ȣ�
Ũ�����������������Ȼ��ˮ��ȣ�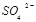 Ũ�� ������ţ���
Ũ�� ������ţ���| A������ | B������ | C������ | D�����ж� |
��8�֣�
��1������¯��ת�������Ӵ��ң����������գ��Ӵ�������� ����
��2����
��
��B
��ÿ��1�֣�
��1������¯��ת�������Ӵ��ң����������գ��Ӵ�������� ����
��2����

��

��B
��ÿ��1�֣�
��1�����Ṥҵ��������Ҫ�豸�з���¯���Ӵ��Һ�������
����������װ�д����Ż����������������գ��Ӵ������
Ũ������������£�SO3���ղ�����ȡ������ʽ
��2������Ȼ��ˮpHԼΪ8��ԭ���Ǻ�ˮ��CO32����HCO3������
������һ������ʽ��2H2SO3��O2=2H2SO4
��SO42��Ũ������ѡB
����������װ�д����Ż����������������գ��Ӵ������
Ũ������������£�SO3���ղ�����ȡ������ʽ
��2������Ȼ��ˮpHԼΪ8��ԭ���Ǻ�ˮ��CO32����HCO3������
������һ������ʽ��2H2SO3��O2=2H2SO4
��SO42��Ũ������ѡB

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 CO2+H2��t��ʱ����1L�ܱ������г���0.2mol CO��0.3molˮ��������Ӧ����ƽ�����ϵ��c(H2)=0.12mol��L-1�����¶��´˷�Ӧ��ƽ�ⳣ��K=_____�������������
CO2+H2��t��ʱ����1L�ܱ������г���0.2mol CO��0.3molˮ��������Ӧ����ƽ�����ϵ��c(H2)=0.12mol��L-1�����¶��´˷�Ӧ��ƽ�ⳣ��K=_____�������������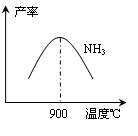
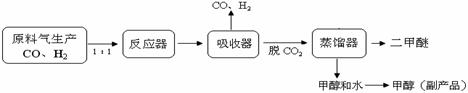
 CH3OH��g�� ��H = ��90.7kJ��mol��1
CH3OH��g�� ��H = ��90.7kJ��mol��1 CH3OCH3��g��+CO2��g������÷�Ӧ�ġ�H =
CH3OCH3��g��+CO2��g������÷�Ӧ�ġ�H = 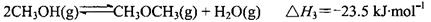
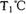 �������ܱ������н�������ƽ�⣬��ϵ�и����Ũ����ʱ��仯��ͼ��ʾ��
�������ܱ������н�������ƽ�⣬��ϵ�и����Ũ����ʱ��仯��ͼ��ʾ��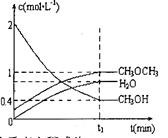
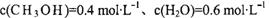 ��
��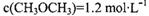 ��ʱ�����淴Ӧ���ʵĴ�С��
��ʱ�����淴Ӧ���ʵĴ�С��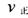 _______
_______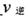 (�>������<������=��)��
(�>������<������=��)��