��Ŀ����
(��16��) I�����Ϊ5 L�ĺ��¡������ܱ���������ʼͶ��2 mol N2 3 mol H2����10s��ƽ�⣬���ƽ��ʱNH3�����ʵ���Ϊ0.8 mol��
II����������ʼͶ��a mol N2��b mol H2��ά�ֺ��¡���ѹ��ƽ�⣬���ƽ��ʱNH3�����ʵ���Ϊ1.2 mol����ƽ���¶���ͬ����ͬ��ֵ������������ͬ��
��1��������10 s ����H2��ʾ��ƽ����Ӧ����v(H2) = _____����ƽ��ʱN2��ת����= ____��
��2��������Щ��������������Ѵ�ƽ��״̬���� ��
��3����ƽ��ʱ��������ѹǿ ��������ѹǿ������ڡ��������ڡ���С�ڡ�����
(4) a = _______________mol��b = ______________mol��
(5) ��ƽ��ʱ�����������Ϊ_____________L��
(6)��ʼʱ��������������ѹǿ��_______________����
II����������ʼͶ��a mol N2��b mol H2��ά�ֺ��¡���ѹ��ƽ�⣬���ƽ��ʱNH3�����ʵ���Ϊ1.2 mol����ƽ���¶���ͬ����ͬ��ֵ������������ͬ��
��1��������10 s ����H2��ʾ��ƽ����Ӧ����v(H2) = _____����ƽ��ʱN2��ת����= ____��
��2��������Щ��������������Ѵ�ƽ��״̬���� ��
| A�������ҵ������ܶȲ��ٱ仯 | B����Ԫ�ص��������ٱ仯 |
| C���������������ʵ��ڰ������������� | D������1 mol N��N��ͬʱ����6 mol N��H�� |
(4) a = _______________mol��b = ______________mol��
(5) ��ƽ��ʱ�����������Ϊ_____________L��
(6)��ʼʱ��������������ѹǿ��_______________����
����16�֣�ÿ��2�֣�
(1) 0.024 mol/(L��s) 20% (2) A D (3) ����
(4) 3 4.5 (5) 7.5 L (6) 0.84
(1) 0.024 mol/(L��s) 20% (2) A D (3) ����
(4) 3 4.5 (5) 7.5 L (6) 0.84
��

��ϰ��ϵ�д�
�����Ŀ
 2HI��������ʾ��ͼ����ʾ�ĺ��������ȷ���ǣ� ��
2HI��������ʾ��ͼ����ʾ�ĺ��������ȷ���ǣ� ��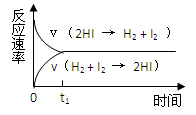
 2C��g������ 2 s ���� C ��Ũ��Ϊ 0.6 mol��L-1 ���������м���˵����
2C��g������ 2 s ���� C ��Ũ��Ϊ 0.6 mol��L-1 ���������м���˵���� 2C(g)��2D��g����H<0����Ӧ���е�10sĩ���ﵽƽ�⣬���A�����ʵ���Ϊ1.8mol��B�����ʵ���Ϊ0.6mol��C�����ʵ���Ϊ0.8mol����
2C(g)��2D��g����H<0����Ӧ���е�10sĩ���ﵽƽ�⣬���A�����ʵ���Ϊ1.8mol��B�����ʵ���Ϊ0.6mol��C�����ʵ���Ϊ0.8mol���� �����䡱��գ���
�����䡱��գ��� 2NH3��
2NH3�� ����N2�����ʵ���Ϊ0.6mol����
����N2�����ʵ���Ϊ0.6mol����
 ��2Z(g)+2W(g)��2L�ܱ������н��У�5min��Y������0.5mol����˷�Ӧ��ƽ������vΪ�� ��
��2Z(g)+2W(g)��2L�ܱ������н��У�5min��Y������0.5mol����˷�Ӧ��ƽ������vΪ�� ��