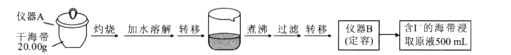
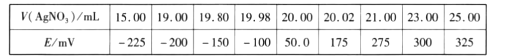
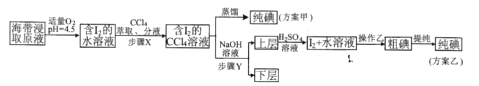
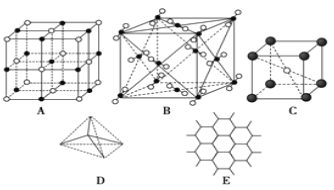
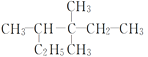��Ŀ����
����Ŀ��Ԫ��A��B��C��D��E��ԭ���������������Ҿ�С��36��A�Ļ�̬ԭ��2p�ܼ���3�������ӣ�C�Ļ�̬ԭ��2p�ܼ���1�������ӣ�D�ļ۵�����Ϊ2����Eͬ���ڣ�E�Ļ�̬ԭ�ӵ��ڲ����ܲ����������4s�ܼ���1�������ӡ��ش��������⣺
(1)��̬Eԭ�ӵļ۵����Ų�ʽΪ____________________
(2)A��B��C����Ԫ�ص�һ�������ɴ�С��˳��Ϊ___________����Ԫ�ط��ű�ʾ����
(3)��A�ĵ��ʷ��ӻ�Ϊ�ȵ�����ķ��Ӻ����ӷֱ���________���÷��Ӻ����ӷ��ű�ʾ����AB2�Ŀռ乹��Ϊ___________������Aԭ�ӵ��ӻ�������_______________
(4)BԪ�ؼ��⻯��ķе���ͬ��Ԫ������ߵģ�ԭ����_____________
(5)��EԪ�ص���������Һ�м��������ˮ���ɵõ�����ɫ����Һ������Һ�м����Ҵ�������������ɫ���塣�þ���Ļ�ѧʽΪ[Cu(NH3)4]SO4H2O�����������ӵĽṹʽΪ____________
(6)C��D�γɻ�����ľ����ṹ��ͼ��ʾ����֪������ܶ�Ϊ��g/cm�������ӵ�����ΪNA�����߳�a=______________cm(�ú�����NA�ļ���ʽ��ʾ����

���𰸡�3d104s1 F��N��O CO��CN-(��C22-) ƽ�������� sp2 B���⻯��ΪH2O��ˮ���Ӽ������� 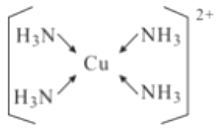
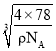
��������
Ԫ��A��B��C��D��E��ԭ���������������Ҿ�С��36��A�Ļ�̬ԭ��2p�ܼ���3�������ӣ�ԭ�Ӻ�������Ų�Ϊ1s22s22p3����A��NԪ�أ�C�Ļ�̬ԭ��2p�ܼ���1�������ӣ���C��ԭ����������A����ԭ�Ӻ�������Ų�Ϊ1s22s22p5������C��FԪ�أ����ԭ����������֪B��OԪ�أ�E�Ļ�̬ԭ�ӵ��ڲ����ܲ����������4s�ܼ���1�������ӣ���ԭ������С��36����E���ڵ������ڣ����̬ԭ�ӵļ۵����Ų�ʽ[Ar]3d104s1����E��CuԪ�أ�D��Eͬ���ڣ��۵�����Ϊ2����D��CaԪ�أ��ݴ˽��
(1)���ݷ�����֪E��ͭԪ�أ����ݹ���ԭ��֪�����̬ԭ�ӵļ۵����Ų�ʽ[Ar]3d104s1���ʻ�̬Cuԭ�ӵļ۵����Ų�ʽΪ��3d104s1��
(2)ͬһ�����У�Ԫ�صĵ�һ����������ԭ�������������������VA��Ԫ�ص�һ�����ܴ��ڵ�VIA��Ԫ�أ�����A��B��C��Ԫ�ص�һ�������ɴ�С��˳��ΪF��N��O��
(3)A��NԪ�أ�A�ĵ��ʷ���Ϊ����(N2)��2��ԭ�ӹ��ɣ��۵�����Ϊ10����Ϊ�ȵ�����ķ��Ӻ����ӷֱ���CO��CN-��C22-��AB2���ɵ�����ΪNO2��Nԭ�ӵijɼ����Ӷ���Ϊ2���µ��Ӷ���=![]() ��(5-2��2)=0.5����������ŵ��ӶԲ�����������ʱӦ����1���Դ�����Ϊ��������ҲҪռ��һ���¶Ե��ӹ����NO2�ļ۲���Ӷ�=2+1������NO2��VSEPRģ��Ϊƽ�������Σ�Nԭ�ӵ��ӻ�������sp2�ӻ���
��(5-2��2)=0.5����������ŵ��ӶԲ�����������ʱӦ����1���Դ�����Ϊ��������ҲҪռ��һ���¶Ե��ӹ����NO2�ļ۲���Ӷ�=2+1������NO2��VSEPRģ��Ϊƽ�������Σ�Nԭ�ӵ��ӻ�������sp2�ӻ���
(4)B��OԪ�أ�OԪ�ص��⻯��ķе���ͬ��Ԫ������ߵģ�����Ϊˮ���Ӽ����γ����������е���ߣ�
(5)����Ļ�ѧʽΪ[Cu(NH3)4]SO4H2O��������ͭ������NH3֮��Ļ�ѧ��Ϊ��λ�����ṹʽΪ ��
��
(6)C��FԪ�أ�D��CaԪ�أ�F��Ca�γɵĻ�����ΪCaF2���ɾ����ṹ��֪�������а�ɫ����Ŀ=8����ɫ����Ŀ=8��![]() +6��
+6��![]() =4����ɫС��ΪF����ɫ����ΪCa��������=
=4����ɫС��ΪF����ɫ����ΪCa��������=![]() �����������Ϊ��a3=
�����������Ϊ��a3=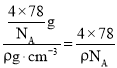 cm3�����Ըþ����߳�a=
cm3�����Ըþ����߳�a=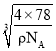 cm��
cm��