��Ŀ����
����Ŀ������ң�C13H18O2)���п��ס���ʹ���������ã���ϳ�·�����£�
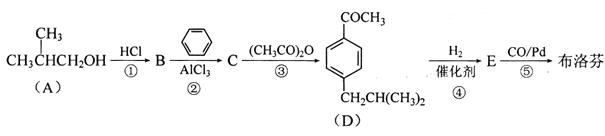
��֪��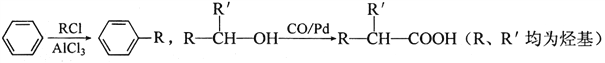
��ش��������⣺
(1)��Ӧ�����У�����ȡ����Ӧ����______ (����)��
(2)E�й����ŵ�����Ϊ______��
(3)����ҵĽṹ��ʽΪ______��
(4)д����Ӧ�۵Ļ�ѧ����ʽ��____________��
(5)�Ȳ������5��̼ԭ�ӵ�ͬϵ��X�ж���ͬ���칹�壬д��ͬʱ�������������� ����
X��ͬ���칹��ṹ��ʽ��_________________��
a.���ڷ������������ b.�˴Ź������׳���4�ַ壬�ҷ����֮��Ϊ3:2:2:1
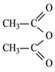
д������һ���ܷ���������Ӧ��������NaOH��Һ��ˮ��ķ�Ӧ����ʽ��______��
���𰸡� �٢ڢ� �ǻ� 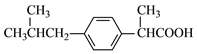
 ��
�� ����
���� ��CH3COOH[
��CH3COOH[ 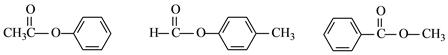
![]() ��2NaOH����
��2NaOH����![]() ��HCOONa��H2O
��HCOONa��H2O
������������ң�C13H18O2)�ϳɹ������漰���ķ�ӦΪ��ȡ����Ӧ ��ȡ����Ӧ
��ȡ����Ӧ
 ��ȡ����Ӧ
��ȡ����Ӧ
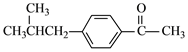
![]() ��
�� ����
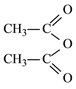
����![]() ��CH3COOH���ܼӳɷ�Ӧ
��CH3COOH���ܼӳɷ�Ӧ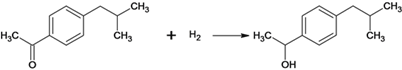 ��
��
(1)��Ӧ�����У�����ȡ����Ӧ�����٢ڢۣ�(2)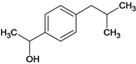 E�й����ŵ�����Ϊ�ǻ���(3)����ҵĽṹ��ʽΪ
E�й����ŵ�����Ϊ�ǻ���(3)����ҵĽṹ��ʽΪ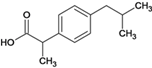 ��(4)��Ӧ��ȡ����Ӧ����ѧ����ʽ��
��(4)��Ӧ��ȡ����Ӧ����ѧ����ʽ�� ��
�� ����
����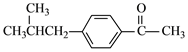 ��CH3COOH��(5) �Ȳ������5��̼ԭ�ӵ�ͬϵ��X����ʽΪC8H8O2��ͬʱ���������������У�
��CH3COOH��(5) �Ȳ������5��̼ԭ�ӵ�ͬϵ��X����ʽΪC8H8O2��ͬʱ���������������У� ������һ���ܷ���������Ӧ��������NaOH��Һ��ˮ��ķ�Ӧ����ʽ��
������һ���ܷ���������Ӧ��������NaOH��Һ��ˮ��ķ�Ӧ����ʽ�� ![]() ��2NaOH����
��2NaOH����![]() ��HCOONa��H2O
��HCOONa��H2O

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ����������ƣ�Na2S2O3)��Ʒ���������������������մ�������ѧ�ϳ����� �ζ�ʵ�顣ij��ѧ��ȤС����ʵ�����Ʊ���������ƾ��岢̽���仯ѧ���ʡ�

I.�Ʊ� Na2S2O3
(1)��ͼ���ر�K1��K2�� ��Ӧ��ʼ��װ��c�еIJ���� �� Na2S2O3���һ����ɫ��ζ �����壬����������______��
(2)װ��c�з�Ӧ������ �ȹرշ�Һ©����������e���� ��ʢNaOH��Һ��ע�������ٹر�K2��K1 ,��Ŀ����_______________����c����Һ��ȴ����������ͨ��������������ȴ�ᾧ����Ȳ������Na2S2O3 CH2O���塣
(3)ʵ�������װ��d�е����ʿ�����__________________��
̽��Na2S2O3�IJ��ֻ�ѧ����
������������Na2S2O3���Կ�����һ��Sԭ��ȡ���� Na2SO4��һ��Oԭ���γɵġ��ݴ� �Ʋ⣬����˵����ȷ����____________��
A.![]() ��
��![]() ������������ṹ B.
������������ṹ B.![]() ��������ļ�������������
��������ļ�������������
C.![]() �еļ��Ǿ�Ϊ
�еļ��Ǿ�Ϊ![]() D.
D.![]() ������ԭ�Ӷ�����8���ӽṹ
������ԭ�Ӷ�����8���ӽṹ
��������衿��Na2S2O3��Na2SO4�ṹ���ƣ���ѧ����ҲӦ�����ƣ��������ʱNa2S2O3 ��ҺpH=7���ڴ�SԪ�صĻ��ϼ��Ʋ�Na2S2O3���н�ǿ�Ļ�ԭ�ԡ�
����֤���衿��������Na2S2O3��Һ����������ʵ�飨�����):
ʵ����� | ʵ������ | ������ͣ������ӷ���ʽ��ʾ�� | |
�� | ___________________ | ��ҺpH=8 | ___________ |
�� | ��������ˮ�е�������Na2S2O3��Һ | ��ˮ��ɫ | _____________ |
��ʵ����ۡ�__________________
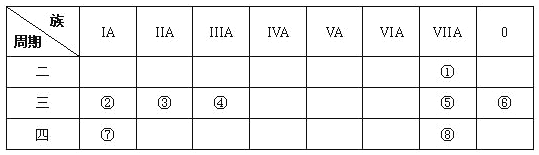
����Ŀ�����ǵ����Ϻ����ḻ��һ��Ԫ�أ����̵����ڹ�ũҵ������������Ҫ���ã��Ǽ���������ѧ��һֱ�о��Ŀ��⡣�±��о��˲�ͬ�¶��´����̵���ҵ�̵��IJ���Kֵ��
��Ӧ | �����̵� N2(g)+O2(g) | ��ҵ�̵� N2(g)+3H2(g) | |||
�¶�/�� | 27 | 2000 | 25 | 400 | 450 |
K | 3.84��10-31 | 0.1 | 5��10-8 | 2��104 | 7��103 |
��1���ٷ������ݿ�֪�������̵���Ӧ����___________������ȡ����ȡ�����Ӧ��
����һ���¶��£���һ�����N2��O2ͨ�뵽���Ϊ1L���ܱ������У����������̵�����Ӧ�ﵽƽ��ı�������������ʹƽ��������Ӧ�����ƶ���ƽ�ⳣ���������_______��
a������ѹǿ b������Ӧ���Ũ�� c��ʹ�ô��� d�������¶�
�� �ӷ��ӽṹ�ǶȽ��͡������̵����͡���ҵ�̵�����Ӧ�Ļ�ܶ��ܸߵ�ԭ��________��
��2�������������ݿ�֪�������̵����ķ�Ӧ������еij̶�С�����ʺϴ��ģ��������������������úϳɰ��ķ������й�ҵ�̵���
�ٴ�ƽ���ƶ��Ƕȿ��ǣ���ҵ�̵�Ӧ��ѡ������������ʵ�ʹ�ҵ����ȴѡ��500�����ҵĸ��£�������ԭ��_______________________��
�ڽ�0.1molN2��0.1molH2ͨ��һ�ݻ��ɱ�������н��й�ҵ�̵���Ӧ������ͼ��ʾN2��ƽ��ת�����ڲ�ͬѹǿ(P1��P2)�����¶ȱ仯��������ȷ����____________���A�� ��B��)���Ƚ�P1��P2�Ĵ�С��ϵ________����300�桢ѹǿP2ʱ�ﵽƽ�⣬�����ݻ�ǡΪ100L�����״̬�·�Ӧ��ƽ�ⳣ��K=______________ (����������2λ��Ч����)��

�ۺϳɰ���Ӧ�ﵽƽ���t1ʱ�̰���Ũ��������ͼC�仯�ɲ�ȡ�Ĵ�ʩ��____________��

��3��������꣬���п�ѧ������ڳ��¡���ѹ�������������ºϳɰ�������˼·����Ӧԭ��Ϊ��2N2(g)+6H2O(l)4NH3(g)+3O2(g)�����䷴Ӧ�ȡ�H=____________��
����֪��N2(g)+3H2(g)2NH3(g) ��H1=-92.4kJmol-1��2H2(g)+O2(g)2H2O(l) ��H2=-571.6kJmol-1 ��