��Ŀ����
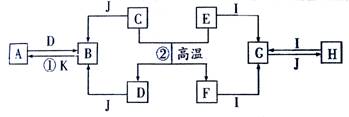
����A��J������ѧ��ѧ�г������ʣ�A��B��C��E��H�dz������壬E������ù�̬��D����٢��ǹ�ҵ�����е���Ҫ��Ӧ�����ǵIJ��ֹ�ϵ��ͼ��ʾ���Իش��������⣺
��1��I�Ļ�ѧʽΪ��________________��
��2����Ӧ�ٵ����ӷ���ʽ��_______________________________________��
��3��B��������E������I����һ�ֳ������ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ������������ת�Ʒ������Ŀ��__________________________��
��4��Ϊ��֤��Ӧ�ڵ�˳�����У����õ��㹻����J��Ӧ��ȡ�Ĵ�ʩ�ǣ�д��������������
��_______________________________����_______________________________��
��1��NH4Cl
��2��2Cl-+2H2O![]() 2OH-+H2��+Cl2��
2OH-+H2��+Cl2��
![]()
��4�����Ȼ�����Һ���뱥��
����ʳ��ˮ����ͨ������������ͨ�������Ķ�����̼����

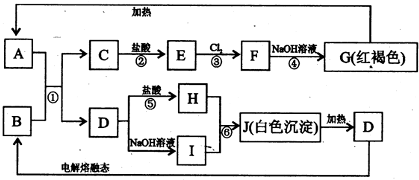
 ��ǰ����ϵ�д�
��ǰ����ϵ�д���8�֣�������A��K����ѧ��ѧ�еij������ʣ�����D��E��KΪ���ʣ���������Ϊ�������Щ���ʾ�������ת����ϵ��ʡ����ˮ�Ͳ��ַ�Ӧ�P�����������Ӧ���⣬������Ӧ������Һ�н��С�D�ǹ�ҵ����õĽ�����
��1��A��H�ֱ�Ϊ �� ���ѧʽ��
��2��д����Ӧ�ڵĻ�ѧ����ʽ ��
��3��2��7��E��I��ȫ��Ӧ����G��G��J��Ӧ����3��9��H ��������J�����ʵ���Ϊ ��
��4��ʵ��ָ������ˮH�ڳ�ѹ�����²�ͬ�¶�ʱ���ܶ�Ϊ��
|
T(��) |
200 |
600 |
800 |
|
��(g/L) |
6��881 |
2��650 |
1��517 |
|
����Ħ�������L/mol�� |
38��8 |
71��6 |
88��0 |
�ɼ���600��ʱ��ˮH ������ʽ�ķ���ʽΪ ��
 ���壬E������ù�̬��D����١����ǹ�ҵ�����е���Ҫ��Ӧ�����ǵIJ��ֹ�ϵ��ͼ��ʾ��
���壬E������ù�̬��D����١����ǹ�ҵ�����е���Ҫ��Ӧ�����ǵIJ��ֹ�ϵ��ͼ��ʾ��

 ���壬E������ù�̬��D����١����ǹ�ҵ�����е���Ҫ��Ӧ�����ǵIJ��ֹ�ϵ��ͼ��ʾ��
���壬E������ù�̬��D����١����ǹ�ҵ�����е���Ҫ��Ӧ�����ǵIJ��ֹ�ϵ��ͼ��ʾ��