��Ŀ����
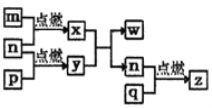
����Ŀ��ij��ѧС��ģ�⡰�����Ƽ������NaCl��NH3��CO2��ˮ��Ϊԭ���Լ���ͼ��ʾװ����ȡNaHCO3����Ӧ�Ļ�ѧ����ʽΪNH3+CO2+H2O+NaCl = NaHCO3��+NH4Cl��Ȼ���ٽ�NaHCO3�Ƴ�Na2CO3��
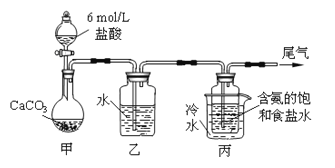
��װ���ҵ�������__________��Ϊ��ֹ��Ⱦ������β���к��е�_____��Ҫ�������մ�����
����װ�ñ��в�����NaHCO3��ȡNa2CO3ʱ����Ҫ���е�ʵ�������__________��NaHCO3ת��ΪNa2CO3�Ļ�ѧ����ʽΪ___________��
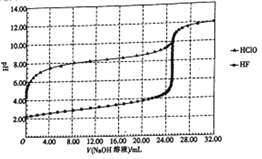
�����ڢ������յ�ʱ��϶̣�NaHCO3���ֽⲻ��ȫ����С���һ�ݼ�����t1min��NaHCO3��Ʒ����ɽ���������̽����
ȡ������t1min��NaHCO3��Ʒ29.6 g��ȫ����ˮ�Ƴ���Һ��Ȼ�������Һ�л����صμ�ϡ���ᣬ�����Ͻ��衣��������ļ��룬��Һ���й����ӵ����ʵ����ı仯����ͼ��ʾ��

������c��Ӧ����Һ�е�������______�������ӷ��ţ�������Ʒ��NaHCO3��Na2CO3�����ʵ���֮����_____��
����ȡ21.0 g NaHCO3���壬������t2min��ʣ����������Ϊl4.8 g������Ѵ�ʣ�����ȫ�����뵽200 mL 2 mol��L-1�����������ַ�Ӧ����Һ��H+�����ʵ���Ũ��Ϊ_________������Һ����仯���Բ��ƣ���
���𰸡���1����ȥ������̼�е��Ȼ������壨2�֣� ���� ��1�֣�
��2�����ˡ�ϴ�ӡ����� ����1�֣� 2NaHCO3![]() Na2CO3+H2O+CO2����2�֣�
Na2CO3+H2O+CO2����2�֣�
��3��HCO3����2�֣� 1:2 ��2�֣� ��4�� 0.75 mol/L��2�֣�
�������������������1��װ�ü����Ʊ�������̼����ķ�Ӧװ�ã����ɵĶ�����̼�����к����Ȼ������壬���Ʊ�̼��������Ӱ�죬װ���ҵ������������Ȼ������壻����β���к��а��������ŷŵ������У���Ҫ����β�����գ��ʴ�Ϊ������HCl��NH3����2����װ�ñ��в�����NaHCO3�����ķ�ӦΪ��NH3+CO2+H2O+NaCl=NaHCO3��+NH4Cl����ȡNa2CO3ʱ��Ҫ���˵õ����壬ϴ�Ӻ�������յõ�̼���ƣ��ʴ�Ϊ�����ˡ�ϴ�ӡ����գ���3�����ڣ�2�������յ�ʱ��϶̣�NaHCO3���ֽⲻ��ȫ����С���һ�ݼ�����t1min��NaHCO3��Ʒ����ɽ������о���ȡ������t1min��NaHCO3��Ʒ29.6g ��ȫ����ˮ�Ƴ���Һ��Ȼ�������Һ�л����صμ�ϡ���ᣬ�����Ͻ��裮��������ļ��룬������Ӧ CO32-+H+=HCO3-�� HCO3-+H+=CO2��+H2O����Һ���й����ӵ����ʵ����ı仯Ϊ̼������Ӽ�С��̼���������Ũ������̼�������ȫ��ת��Ϊ̼��������ӣ��ٵ��������̼��������ӷ�Ӧ���ɶ�����̼��̼��������Ӽ�С������c���߱�ʾ����̼���������Ũ�ȱ仯��̼�������Ũ��0.2mol/L��̼���������Ũ��Ϊ0.1mol/L����Ʒ��NaHCO3��Na2CO3�����ʵ���֮����1��2���ʴ�Ϊ��HCO3-�� 1��2����4����ȡ21g NaHCO3�������ʵ���Ϊ0.25mol ��������t2min��ʣ����������Ϊ14.8g�����ݻ�ѧ����ʽ���ڵ������仯���㣺 2NaHCO3=Na2CO3+CO2��+H2O��m
2 1 62
0.2mol 0.1mol 21g-14.8g
��Ӧ��NaHCO3���ʵ���=0.25mol-0.2mol=0.05mol��NaHCO3+HCl=NaCl+H2O+CO2���������Ȼ������ʵ���0.05mol�� Na2CO3���ʵ���=0.1mol��Na2CO3+2HCl=2NaCl+H2O+CO2���������Ȼ������ʵ���0.2mol��ʣ���Ȼ������ʵ���=0.200L��2mol/L-0.05mol-0.2mol=0.15mol��ʣ����Һ��c��H+��=0.75mol/L

����Ŀ��������ʯ����Ҫ�ɷ�ΪFeS2������FeS���������������в���Fe��SԪ�أ��Ҹ����²�������ѧ�仯�������ҹ���������᳧��ȡ�������Ҫԭ�ϡ�ij��ѧ��ȤС��Ըû�����ʯ��������ʵ��̽������m 1 g�û�����ʯ����Ʒ��������ͼװ�ã��гֺͼ���װ���ԣ���ʯӢ���У���a�����ϵػ���ͨ��������������ջ�������Ʒ����Ӧ��ȫ���䷴Ӧ�Ļ�ѧ����ʽΪ��
4FeS2+11O2![]() 2Fe2O3+8SO2 4FeS+7O2
2Fe2O3+8SO2 4FeS+7O2![]() 2Fe2O3+4SO2
2Fe2O3+4SO2
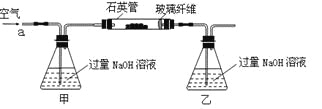
��ʵ��һ�����ⶨ��Ԫ�صĺ�����Ӧ��������ƿ�е���Һ�������´�����
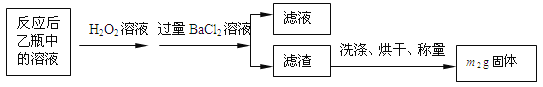
��1�����������������__________________________________________��
��2����Ӧ��������ƿ�е���Һ�������H2O2��Һ��Ŀ����_______________���û�ѧ����ʽ��ʾ����
H2O2���Կ�����һ�ֺ������ᣬд������뷽��ʽ�� ��
��3���û�����ʯ����Ԫ�ص���������Ϊ__________���г�����ʽ���ɣ���
��ʵ��������ⶨ��Ԫ�صĺ���
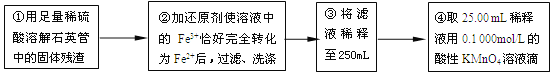
��4�����У�������������ԭ����������õ���Ԫ�صĺ���_______������ƫ��������ƫС��������Ӱ��������
��5�����У���Ҫ�õ����������ձ�������������ͷ�ι��⣬����_______��
��6��ijͬѧһ���������Ĵεζ�ʵ�飬ʵ������¼���£�
ʵ����� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
����KMnO4��Һ���/mL | 25.00 | 25.03 | 20.00 | 24.97 |
�����������ݣ������ϡ��Һ��Fe2+�����ʵ���Ũ��Ϊc(Fe2+) ��___________��