��Ŀ����
1����һ�������£���ϩ�����鶼�������Ʊ������飮��ش��������⣺��1���������Ʊ�������Ļ�ѧ����ʽΪ��CH3CH3+Cl2$\frac{\underline{\;����\;}}{\;}$CH3CH2Cl+HCl���÷�Ӧ����ȡ����Ӧ��
��2������ϩ�Ʊ�������Ļ�ѧ����ʽΪ��CH2=CH2+HCl$\stackrel{һ������}{��}$CH3CH2Cl���÷�Ӧ���ڼӳɷ�Ӧ��
��3���Ƚ����ַ�����֪���ڣ�2��[���1������2����]�ַ����õ��IJ����������
���� ����Ϊ���������������ڹ��������·���ȡ����Ӧ�������ɶ����ȴ�������ϩ��HCl�ڴ������¿ɷ����ӳɷ�Ӧ���Դ˽��
��� �⣺��1������Ϊ���������������ڹ��������·���ȡ����Ӧ������������ķ���ʽΪCH3CH3+Cl2 $\frac{\underline{\;����\;}}{\;}$CH3CH2Cl+HCl��
�ʴ�Ϊ��CH3CH3+Cl2 $\frac{\underline{\;����\;}}{\;}$CH3CH2Cl+HCl��ȡ����Ӧ��
��2����ϩ��HCl�ڴ������¿ɷ����ӳɷ�Ӧ������ʽΪCH2=CH2+HCl$\stackrel{һ������}{��}$ CH3CH2Cl��
�ʴ�Ϊ��CH2=CH2+HCl$\stackrel{һ������}{��}$ CH3CH2Cl���ӳɷ�Ӧ��
��3���������������ڹ��������·�����Ӧ�õ��IJ����У�һ�����飬1��1-�������飬1��2-�������飬1��1��2-�������飬1��1��2��2-�������飬1��1��1��2-�������飬1��1��1��2��2-�������飬����������Ȼ��⣬���ﲻΨһ������ϩ��±�����ܷ����ӳɷ�Ӧ�õ������飬����ֻ��һ�֣�
�ʴ�Ϊ����2����
���� ���⿼�����л�����Ʊ��ͷ��������ۣ�Ϊ��Ƶ���㣬����ѧ���ķ��������Ŀ��飬��Ŀ�ѶȲ���ע����л���ṹ�����ʵ����գ�

 ��������ܸ�ϰϵ�д�
��������ܸ�ϰϵ�д�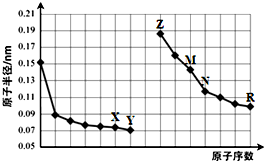
| A�� | X��R����Ԫ�ص���̬�⻯����ȶ��ԣ�R��X | |
| B�� | �����ӵİ뾶��Y��Z��M | |
| C�� | N�ĵ�������Z������������Ӧ��ˮ���ﷴӦ | |
| D�� | Z��X����Ԫ���γɵĻ����ﶼ�Ǽ��������� |
�ٽϸ߷е�
��������ˮ
��ˮ��Һ�ܵ���
���۵�ܵ�
������״̬�����磮
| A�� | �٢ڢ� | B�� | �ۢܢ� | C�� | �٢ܢ� | D�� | �ڢۢ� |
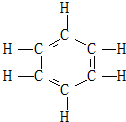 1866�꿭��������˱��ĵ���˫���������������ƽ��ṹ����ͼ���������˱��IJ������ʣ�������һЩ������δ������������ܽ��ͣ�������
1866�꿭��������˱��ĵ���˫���������������ƽ��ṹ����ͼ���������˱��IJ������ʣ�������һЩ������δ������������ܽ��ͣ��������ٱ�����ʹ���CCl4��Һ��ɫ���ڱ�����H2�����ӳɷ�Ӧ
���屽û��ͬ���칹�� ���ڶ��屽ֻ��һ�֣�
| A�� | �٢� | B�� | �� | C�� | �ڢ� | D�� | �� |
| A�� | 4�� | B�� | 3 �� | C�� | 2�� | D�� | 1�� |
| A�� | ��������Һ����μ������Na2SiO3+2H+=H2SiO3��+2Na2+ | |
| B�� | ������������Һ�м��������Һ��OH-+H+=H2O | |
| C�� | ͭ��ϡ���ᷴӦ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O | |
| D�� | ����SO2ͨ��Ca��ClO��2��Һ�У�SO2+H2O+Ca2++2ClO-=CaSO3��+2HClO |
| A�� | $\frac{a}{N}$��N-a��mol | B�� | $\frac{a}{A+16}$��A-N+10��mol | C�� | $\frac{a}{A+8}$��N+n��mol | D�� | $\frac{a}{A+16}$��A-N+8��mol |
| A�� | �����ʵ���Ũ�ȵİ�ˮ������������ϣ�c��Cl-����c��NH4+����c��H+����c��OH-�� | |
| B�� | c��NH4+����ȣ�NH4��2SO4��Һ����NH4��2CO3��Һ��NH4Cl��Һ��c[��NH4��2CO3]��c[��NH4��2SO4]��c[NH4Cl] | |
| C�� | ���ʵ���Ũ����ȵ�CH3COOH��CH3COONa��Һ�������ϣ�c��Na+��+c��OH-��=c��H+��+c��CH3COOH�� | |
| D�� | �����£�NaB��Һ��pH=8��c��Na+��-c��B-��=9.9��10-7mol•L-1 |
 ����Fe��OH��2���ױ�����������ʵ���Һ�������������Һ���ռӦ�Ƶð�ɫ������Fe��OH��2������Ӧ����ͼ��ʾװ�ÿ����Ƶð�ɫ������Fe��OH��2�������������Ϸֱ�Ϊ����ʯī��
����Fe��OH��2���ױ�����������ʵ���Һ�������������Һ���ռӦ�Ƶð�ɫ������Fe��OH��2������Ӧ����ͼ��ʾװ�ÿ����Ƶð�ɫ������Fe��OH��2�������������Ϸֱ�Ϊ����ʯī��