��Ŀ����
����Ŀ���л���J��һ�ַ�ֹѪ����Ѫ˨�γ��뷢չ��ҩ���ϳ�·����ͼ��ʾ(���ַ�Ӧ������ȥ)��

��ش���������:
(1)B������Ϊ______________����Ӧ�ķ�Ӧ������__________��
(2)J����_______�ֹ����š�F�Ľṹ��ʽ��_____________��
(3)��Ӧ�۵Ļ�ѧ����ʽΪ____________________________________________-��
(4)д��ͬʱ��������������F��ͬ���칹��Ľṹ��ʽ:______________(����д����)��
�ٱ�����ֻ���������ڶ�λ��ȡ������
��1 mol���л������뺬2 mol NaOH����Һǡ����ȫ��Ӧ��
(5)����D�ĺϳ�·�ߣ����һ����![]() Ϊԭ���Ʊ�
Ϊԭ���Ʊ� �ĺϳ�·��__________��
�ĺϳ�·��__________��
���𰸡����ᱽ���ӣ��� �ӳɷ�Ӧ 4 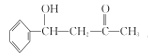

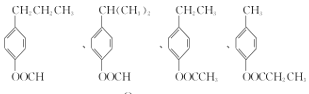

��������
����A����������Ӧ�������ǻ��ϵ���������������ڴ��������£����нṹת������ʱ���ǻ��ָ���������������ǻ���λ�� C��D��ת��������Ϣ���Ѹ������ؾ�����η�Ӧ����дʱ��עԭ���غ㣬�жϳ��������Ҵ����ɡ�
(1)��Bˮ��ɵ�����ͱ��ӣ���ϵͳ��������B������Ϊ���ᱽ����G�Ͽ�̼̼˫����D�ϳ�J����Ӧ������Ϊ�ӳɷ�Ӧ��
(2)J�к����ǻ����ʻ����Ȼ���̼̼˫����4�ֹ����ţ�����F�ķ���ʽ����G�Ľṹ��ʽ���ƿɵ�F�Ľṹ��ʽΪ��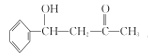 ��
��
(3)����ԭ���غ�����������ķ�Ӧ���벿���������д����Ӧ�۵Ļ�ѧ����ʽΪ�� ��
��
(4) 1 mol���л������뺬2 mol NaOH����Һǡ����ȫ��Ӧ���ұ�����ֻ���������ڶ�λ��ȡ������������������ͬ���칹�����Ƿӣ�F�г�ȥ��������һ�������Ͷȣ������������Ʒ�Ӧ������ֻ�������࣬Ҫ��Ӧ��2mol�������ƣ���Ӧ�Ƿ���������������ͬ���칹��Ľṹ��ʽ�У� ��
��
(5) ԭ�϶Ա�A��һ������Ҫ�ϳɵ����ʶԱ�D��һ�������ο�A�ϳ�D�ķ����ɵã�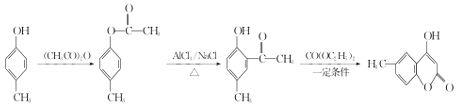 ��
��








