��Ŀ����
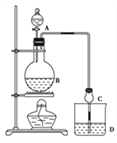
����Ŀ��ij����С����Ƶ�ʵ������ȡ����������װ����ͼ��ʾ��A�з���Ũ���ᣬB�з����Ҵ�����ˮ�����ƣ�D�з��б���̼������Һ��
��֪������ˮ�Ȼ��ƿ����Ҵ��γ�������ˮ��CaCl26C2H5OH
���й��л���ķе㣺
�Լ� | ���� | �Ҵ� | ���� | �������� |
�е�/�� | 34.7 | 78.5 | 118 | 77.1 |
��ش�
��1��Ũ�����������_____________________________������ͬλ��18Oʾ�ٷ�ȷ����Ӧ����ˮ��������ԭ�ӵ��ṩ�ߣ�д���ܱ�ʾ18Oλ�õĻ�ѧ����ʽ_____________________________��
��2�����θ����C��������______________________������Ӧǰ��D�м��뼸�η�̪����Һ�ʺ�ɫ�������������ԭ����______________________����Ӧ������D�е�����______________________��
��3����D�з���������������г�����һ�������Ҵ������Ѻ�ˮ��Ӧ�ȼ�����ˮ�Ȼ��ƣ������___________________��
���𰸡� �����ᡢ��������ˮ�� CH3COOH+CH3CH218OH![]() CH3CO18OC2H5+H2O ��ֹ���������� ̼������Һ�Լ��� ��Һ�ֲ㣬�ϲ���ɫ����Һ�壬�²���Һ��ɫ��dz �Ҵ�
CH3CO18OC2H5+H2O ��ֹ���������� ̼������Һ�Լ��� ��Һ�ֲ㣬�ϲ���ɫ����Һ�壬�²���Һ��ɫ��dz �Ҵ�
�������������������1��Ũ����������Dz������ᡢ��������ˮ����������Ӧ����ˮ��ʽ�ǣ������ǻ������⡣��������ͬλ��18Oʾ�ٷ�ȷ����Ӧ����ˮ��������ԭ�ӵ��ṩ�ߣ��ܱ�ʾ18Oλ�õĻ�ѧ����ʽΪ��CH3COOH��C2H518OH![]() CH3CO18OC2H5��H2O����2�����θ����C�������������ͷ�ֹ������D�з��б���̼������Һ����Ӧǰ��D�м��뼸�η�̪����Һ�ʺ�ɫ�������������ԭ������Ϊ̼������ǿ�������Σ�����Һ�д���ˮ��ƽ�⣺CO32-��H2O
CH3CO18OC2H5��H2O����2�����θ����C�������������ͷ�ֹ������D�з��б���̼������Һ����Ӧǰ��D�м��뼸�η�̪����Һ�ʺ�ɫ�������������ԭ������Ϊ̼������ǿ�������Σ�����Һ�д���ˮ��ƽ�⣺CO32-��H2O![]() HCO3-��OH����HCO3-��H2O
HCO3-��OH����HCO3-��H2O![]() H2CO3��OH�����ƻ���ˮ�ĵ���ƽ�⣬ˮ�������룬���յ���Һ�дﵽƽ��ʱ��c(OH��)��c(H+)��������Һ�Լ��ԡ�����̼������Һ���������ܽ��Ҵ�����Ӧ���Ļӷ��������ᣬ���������������ܽ�ȡ����������������ܶȱ�ˮС�������ܽ���ˮ�����Իῴ����Һ�ֲ㣬�ϲ���ɫ��״Һ�壬�²���Һ��ɫ��dz����3��������ˮ�Ȼ��ƿ����Ҵ��γ�������ˮ��CaCl2��6C2H5OH�������ȼ�����ˮ�Ȼ��ƣ�������Ҵ���
H2CO3��OH�����ƻ���ˮ�ĵ���ƽ�⣬ˮ�������룬���յ���Һ�дﵽƽ��ʱ��c(OH��)��c(H+)��������Һ�Լ��ԡ�����̼������Һ���������ܽ��Ҵ�����Ӧ���Ļӷ��������ᣬ���������������ܽ�ȡ����������������ܶȱ�ˮС�������ܽ���ˮ�����Իῴ����Һ�ֲ㣬�ϲ���ɫ��״Һ�壬�²���Һ��ɫ��dz����3��������ˮ�Ȼ��ƿ����Ҵ��γ�������ˮ��CaCl2��6C2H5OH�������ȼ�����ˮ�Ȼ��ƣ�������Ҵ���