ĢāÄæÄŚČŻ
£Ø10·Ö£©ÓŠA”¢B”¢C”¢D”¢EĪåÖÖ¶ĢÖÜĘŚŌŖĖŲ£¬ĒŅĻąĮŚµÄA”¢B”¢C”¢DĖÄÖÖŌŖĖŲŌ×ÓŗĖĶā¹²ÓŠ56øöµē×Ó£¬ŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆČēĶ¼ĖłŹ¾”£EµÄĒāŃõ»ÆĪļŹĒĮ½ŠŌĒāŃõ»ÆĪļ£¬EµÄŃōĄė×ÓÓėAµÄŅõĄė×ÓŗĖĶāµē×Ó²ć½į¹¹ĻąĶ¬”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
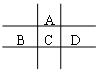
£Ø1£©BŌŖĖŲŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆĪŖ £»
£Ø2£©AÓėĒāŌŖĖŲÄÜŠĪ³ÉŌ×ÓĪļÖŹµÄĮæÖ®±ČĪŖ1:1µÄ»ÆŗĻĪļ£¬Ęäµē×ÓŹ½ĪŖ £»
£Ø3£©ĻņDÓėEŠĪ³ÉµÄ»ÆŗĻĪļµÄĖ®ČÜŅŗÖŠµĪČėNaOHČÜŅŗÖ±ÖĮ¹żĮ棬¹Ū²ģµ½µÄĻÖĻóŹĒ £¬×īŗó½×¶Ī·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ”””””””” £»
£Ø4£©B”¢C”¢D”¢EµÄ×īøß¼ŪŃõ»ÆĪļµÄĖ®»ÆĪļµÄĖįŠŌ“ÓĒæµ½ČõµÄĖ³ŠņĪŖ£ØĪļÖŹÓĆ»ÆѧŹ½±ķŹ¾£© ”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ

£Ø1£©BŌŖĖŲŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆĪŖ £»
£Ø2£©AÓėĒāŌŖĖŲÄÜŠĪ³ÉŌ×ÓĪļÖŹµÄĮæÖ®±ČĪŖ1:1µÄ»ÆŗĻĪļ£¬Ęäµē×ÓŹ½ĪŖ £»
£Ø3£©ĻņDÓėEŠĪ³ÉµÄ»ÆŗĻĪļµÄĖ®ČÜŅŗÖŠµĪČėNaOHČÜŅŗÖ±ÖĮ¹żĮ棬¹Ū²ģµ½µÄĻÖĻóŹĒ £¬×īŗó½×¶Ī·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ”””””””” £»
£Ø4£©B”¢C”¢D”¢EµÄ×īøß¼ŪŃõ»ÆĪļµÄĖ®»ÆĪļµÄĖįŠŌ“ÓĒæµ½ČõµÄĖ³ŠņĪŖ£ØĪļÖŹÓĆ»ÆѧŹ½±ķŹ¾£© ”£
£Ø10·Ö£©
£Ø1£©µŚČżÖÜĘŚ¢õA×å£Ø2·Ö£©
£Ø2£©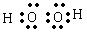 £Ø2·Ö£©
£Ø2·Ö£©
£Ø3£©ĻČ²śÉś°×É«³Įµķ£¬Č»ŗó³ĮµķČܽā£Ø2·Ö£©£¬
Al(OH)3 + OH”Ŗ=AlO2”Ŗ+2H2O£Ø2·Ö£©
£Ø4£©HClO4 >H2SO4 >H3PO4 >Al(OH)3 £Ø2·Ö£©
£Ø1£©µŚČżÖÜĘŚ¢õA×å£Ø2·Ö£©
£Ø2£©
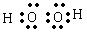 £Ø2·Ö£©
£Ø2·Ö£©£Ø3£©ĻČ²śÉś°×É«³Įµķ£¬Č»ŗó³ĮµķČܽā£Ø2·Ö£©£¬
Al(OH)3 + OH”Ŗ=AlO2”Ŗ+2H2O£Ø2·Ö£©
£Ø4£©HClO4 >H2SO4 >H3PO4 >Al(OH)3 £Ø2·Ö£©
ĀŌ

Į·Ļ°²įĻµĮŠ“š°ø
 Š”ѧ½Ģ²ÄČ«²āĻµĮŠ“š°ø
Š”ѧ½Ģ²ÄČ«²āĻµĮŠ“š°ø Š”ѧŹżŃ§æŚĖćĢāæØĶŃæŚ¶ų³öĻµĮŠ“š°ø
Š”ѧŹżŃ§æŚĖćĢāæØĶŃæŚ¶ų³öĻµĮŠ“š°ø
Ļą¹ŲĢāÄæ
 v
v