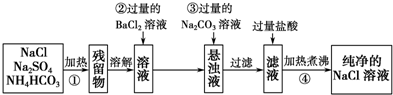
��Ŀ����
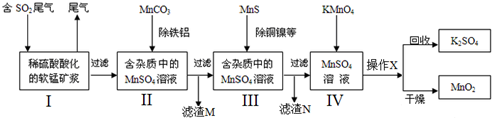
9���״���һ����Ҫ���л�����ԭ�ϣ���������Ŀǰ�ҹ��������������յĺ����Dz���һ����̼������ѹ�ϳɷ�����Ҫ��Ӧ���£�CO��g��+2H2��g���TCH3OH��g������H=-111.0kJ/mol���и���Ӧ��2CO+2H2O�TCO2+CH4 �ȣ���ѹ��������ѹ��Ϊ10-15MPa���¶ȿ�����513K-543K�����ô�����CuO-ZnO-Al2O3���ϳɼ״���������ͼ��ʾ��
��ش�
��1��ʵ��������CO���ܹ��������������ʻ���ʹ����ʧȥ���ԣ�����������������ģ�ָ���������ɣ��ȿ��Է�ֹ����ٸ���Ӧ�������ֿɴ��߷�Ӧ�ȱ���������ȶ��ж���
��2����ȡ10-15MPaѹ���������Ǽ�ѹ���������COת���ʣ���Ҳ��������Դ���ĺ��豸ǿ�ȣ����˲�ȡ����Ч��Ϻõ�ѹ�����¶ȿ�����513K-543K��ԭ���Ǵ��¶��´������Խϸߣ��״����ʽϴ�
��3��ԭ�����е�H2S��ͭ����Ӱ�����أ��ʱ���ȥ��֮��ͨ������ʯ�ҳ��ӣ��÷�Ӧ�Ļ�ѧ����ʽΪH2S+CaO=CaS+H2O��
��4���ּ״��к��ж����ѵȸ�����ڳ�ѹ������ּ״������Ȼ�õ������CH3OCH3��д�ṹ��ʽ������õļ״��ֿ��������������ɼ�ȩ���÷�Ӧ�Ļ�ѧ����ʽΪ��2CH3OH+O2 $��_{��}^{����}$2HCHO+2H2O��
��5����CO��ת����Ϊ80%������22.4M3�������CO�����H2��ַ�Ӧ��������������Ӧ�������Ƶô���Ϊ96%�ļ״�������Ϊ26.67kg��ͬʱ�������8.88��104kJ��
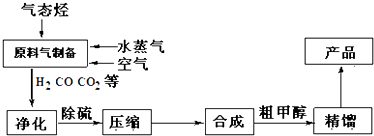
���� ��̬����ˮ�����Ϳ����Ʊ�������һ����̼��������̼������Ȼ�����壬ͨ��������ȥ������̼�õ�������һ����̼�����⣬Ȼ���ȥ���⣬���յõ�������̼�������Ļ�����壬��ѹ����Һ̬��һ����̼��������Ȼ���ںϳ����кϳɵõ��ֲ�Ʒ��������ü״���
��1�������������һ����̼��ת���ʣ���ֹ����Ӧ�����������������ֿɴ��߷�Ӧ������
��2��CO��g��+2H2��g���TCH3OH��g������H=-111.0kJ/mol������Ӧ�����������С�ķ�Ӧ������ѹǿ��Ȼ������ƽ�������ƶ�����״��IJ�������ѹ������豸��ǿ��Ҫ��߳ɱ�������Ӧ�Ƿ��ȷ�Ӧ���������ڼ״����ɵĽǶ�Ӧ�ǵ��£����¶ȹ��ʹﲻ�������Ļ��ԣ�
��3��ԭ�����е�H2S��ͭ����Ӱ�����أ��ʱ���ȥ��֮��ͨ������ʯ�ҳ��ӣ������ƺ�ˮ�����Է�Ӧ�Ļ�ѧ����ʽΪH2S+CaO=CaS+H2O��
��4���״����Ӽ������������Էе���ڶ����ѣ��������Ȼ�õ�����Ƕ����ѣ����Ĵ�����ʵ�������⣬���Է�Ӧ����ʽΪ��2CH3OH+O2 $��_{��}^{����}$2HCHO+2H2O��
��5������ CO��g��+2H2��g���TCH3OH��g������1mol��һ����̼����32��10-3kg�ļ״���22.4M3������������ʵ���Ϊ��$\frac{22.4��1{0}^{3}}{22.4}$=103mol������CO��g��+2H2��g���TCH3OH��g������H=-111.0kJ/mol��1molCO��ȫ��Ӧ�ų�111KJ���������ɴ˷������
��� �⣺��1�������������һ����̼��ת���ʣ���ֹ����Ӧ�����������������ֿɴ��߷�Ӧ������
�ʴ�Ϊ���ȿ��Է�ֹ����ٸ���Ӧ�������ֿɴ��߷�Ӧ�ȱ���������ȶ��ж���
��2��CO��g��+2H2��g���TCH3OH��g������H=-111.0kJ/mol������Ӧ�����������С�ķ�Ӧ������ѹǿ��Ȼ������ƽ�������ƶ�����״��IJ�������ѹ������豸��ǿ��Ҫ��߳ɱ������Լ�ѹ���������COת���ʣ���Ҳ��������Դ���ĺ��豸ǿ�ȣ����˲�ȡ����Ч��Ϻõ�ѹ��������Ӧ�Ƿ��ȷ�Ӧ���������ڼ״����ɵĽǶ�Ӧ�ǵ��£����¶ȹ��ʹﲻ�������Ļ��ԣ����Դ��¶��´������Խϸߣ��״����ʽϴ�
�ʴ�Ϊ����ѹ���������COת���ʣ���Ҳ��������Դ���ĺ��豸ǿ�ȣ����˲�ȡ����Ч��Ϻõ�ѹ�������¶��´������Խϸߣ��״����ʽϴ�
��3��ԭ�����е�H2S��ͭ����Ӱ�����أ��ʱ���ȥ��֮��ͨ������ʯ�ҳ��ӣ������ƺ�ˮ�����Է�Ӧ�Ļ�ѧ����ʽΪH2S+CaO=CaS+H2O��
�ʴ�Ϊ��H2S+CaO=CaS+H2O��
��4���״����Ӽ������������Էе���ڶ����ѣ��������Ȼ�õ�����Ƕ����ѣ����Ĵ�����ʵ�������⣬���Է�Ӧ����ʽΪ��2CH3OH+O2 $��_{��}^{����}$2HCHO+2H2O���ʴ�Ϊ��CH3OCH3��2CH3OH+O2 $��_{��}^{����}$2HCHO+2H2O��
��5������ CO��g��+2H2��g���TCH3OH��g������1mol��һ����̼����32��10-3kg�ļ״���22.4M3������������ʵ���Ϊ��$\frac{22.4��1{0}^{3}}{22.4}$=103mol�����Կ��Ƶô���Ϊ96%�ļ״�������Ϊ$\frac{1{0}^{3}��80%��32��1{0}^{-3}}{96%}$=26.67kg������CO��g��+2H2��g���TCH3OH��g������H=-111.0kJ/mol��1molCO��ȫ��Ӧ�ų�111KJ��������103mol��80%=800mol�����Էų�����Ϊ��111��800=8.88��104kJ���ʴ�Ϊ��26.67kg��8.88��104kJ��
���� ���⿼������������ڻ�ѧ��Ӧ���ʡ��л���ѧ��Ӧ���̵���д����ؼ��㣬���ջ����ǽ���Ĺؼ�����Ŀ�Ѷ��еȣ�

| A�� | ���ۡ���ά�ء���֬�������ʶ�������Ȼ�л��߷��ӻ����� | |
| B�� | ʵ����ֻ���Ҵ��������ϼ��ȾͿ����Ƶ��������� | |
| C�� | ���������Ƶ�������ͭ����Һ������Һ�е������� | |
| D�� | CH3CH2CH��CH3��CH3��CH3CH��CH3��CH2CH3��Ϊͬ���칹�� |
| A�� | ������������������������ԭ��Ӧ�������� | |
| B�� | �廯������ȶ��Աȵ⻯������ȶ��Բ� | |
| C�� | KI������Һ����ˮ��Ӧ��֤����Ľ����Աȵ�Ľ���ǿ | |
| D�� | I2O5��Br2O5��ˮ��Ӧ���ɶ�Ӧ�ĸ�±�� |
| A�� | ʯ�ͷ����ܵõ�ʯ���������ͺ�ú�͵������Դ | |
| B�� | 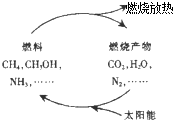 ����ͼѭ��ת�������У�̫��������ת��Ϊ���� | |
| C�� | ú�ĸ�����ָ��ú�ڿ����м��ȵĹ��̣���ҵ��Ҳ��ú�Ľ��� | |
| D�� | ���ࡢ��֬����������Ϊ������������ṩ�����Ļ���Ӫ�����ʣ����ܷ���ˮ�ⷴӦ |
| A�� | ����̼ԭ���������ӣ��������ۡ��е����� | |
| B�� | ���л����У�̼ԭ��ͨ���ĸ����ۼ�������ԭ�ӽ�� | |
| C�� | ���顢��ϩ����ʹ���Ը��������Һ��ɫ | |
| D�� | �����ڹ���������������ȡ����Ӧ |
| Ԫ�ش��� | K | L | M | Q | R | T | N |
| ԭ�Ӱ뾶/nm | 0.183 | 0.160 | 0.143 | 0.102 | 0.089 | 0.074 | 0.152 |
| ��Ҫ���ϼ� | +1 | +2 | +3 | +6��-2 | +2 | -2 | +1 |
| A�� | K��L��M����Ԫ�صĽ����������� | |
| B�� | ��RCl2�����У���ԭ�Ӿ�����8���ӵ��ȶ��ṹ | |
| C�� | QԪ�ص����������Ϊ����ʣ���ˮ��Һ�ܹ����� | |
| D�� | K��T������ȼ�����γɵĻ�����������������������Ŀ֮��Ϊ2��1 |
| A�� | ���Ӷ�Ƥ���и�ʴ�� | B�� | �Ҵ��Dz�����������Դ | ||
| C�� | ��������75%�ľƾ�Ϊҽ�þƾ� | D�� | ��Ȼ����Ҫ�ɷ��������ϩ |