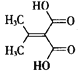
��Ŀ����
����Ŀ�������£�������������Һ��
�� | �� | �� | �� | �� |
0.1 mol��L-1 CH3COOH��Һ | 0.01mol��L-1 CH3COOH��Һ | pH��2 CH3COOH��Һ | 0.1 mol��L-1 NaOH��Һ | 0.1mol��L-1 ��ˮ |
�ش��������⣺
(1)��Һ��ϡ�͵�ԭ����10�������ҺpH______����Һ��pH������������������������������ͬ��������������Һ��ˮ�������c(H��)����_______��
(2)����ͬ�¶�ʱ100mL ������Һ��10mL ������Һ��Ƚϣ�������ֵǰ�ߴ��ں��ߵ���_______ ������ĸ����
A ���к�ʱ����NaOH���� B ������̶�
C ��ˮ�������c(H��) D�� CH3COOH�����ʵ���
(3)��ˮϡ����ʱ����Һ������ˮ�������Ӷ���С����______������ĸ����
A��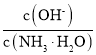 �������������� B��
�������������� B��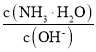
C�� c��H+����c��OH-���ij˻������� D��OH-�����ʵ���
(4)������N2H4������ˮ�Լ��ԣ���ԭ���백���ƣ�������Բ��簱ǿ��д��������ˮ�ʼ��Ե����ӷ���ʽ��_______________________________________��
(5)��֪CH3COOH�ĵ���ƽ�ⳣ��Ka=1.8��10��5 ����һ�ֶ�Ԫ����H2B�ĵ���ƽ�ⳣ��ΪK1=4.3��10- 4 ��K2=5.6��10-1 1 ��д����Na2B��Һ�м������CH3COOH��Һ�Ļ�ѧ����ʽ��_________________________________________��
���𰸡�> > BC B N2H4+H2O![]() NH2NH3++OH-����N2H4+H2O
NH2NH3++OH-����N2H4+H2O![]() N2H5++OH-����NH2NH3++H2O
N2H5++OH-����NH2NH3++H2O![]() NH3NH32++OH-����NH2NH3++H2O
NH3NH32++OH-����NH2NH3++H2O![]() N2H62++OH-��N2H5++H2O
N2H62++OH-��N2H5++H2O![]() N2H62++OH-�� CH3COOH+Na2B=CH3COONa+NaHB
N2H62++OH-�� CH3COOH+Na2B=CH3COONa+NaHB
��������
,
��1�����д���Ũ��Ϊ0.1mol/L����ҺpH>2��ϡ��10������ҺpH���ɴ���2����ϡ�͵�ԭ����10�����۵�������Ũ�ȴ�
����0.1molL-1 CH3COOH��Һ���ֵ��룬����0.1molL-1NaOH��Һ��ȫ���룬�����������ƶ�ˮ�����Ƴ̶ȴ��ڴ�����Һ������ˮ����������ӵ�Ũ�Ȣ�>�ڣ�
�ʴ�Ϊ��>��>��
��2������ͬ�¶�ʱ��100mL�ڵ���Һ��10mL�ٵ���Һ��Ƚϣ�
A�����ߴ����������������ʵ�����ȣ�����к�ʱ����NaOH������ȣ��ʲ�ѡ��
B��ԽϡԽ���룬��ˢ��д������̶ȴ��ڢ��д������̶ȣ���ѡ��
C��������Һ��������Ũ�ȴ���ǰ�ߣ�����ˮ�������c(H+)ǰ�ߴ��ں��ߣ���ѡ��
D����ʼCH3COOH�����ʵ���������ȣ���Ϊǰ�ߵĵ���̶�С�ں��ߣ�������Һ������CH3COOH�����ʵ���ǰ��С�ں��ߣ��ʲ�ѡ��
��ѡBC��
��3��ϡ�����У�NH3��H2O�ĵ���̶�������Һ��n(OH-)��n(NH4+)����n(NH3H2O)��С��
A�� =
= �����ֵ������
�����ֵ������
B�� =
= �����ֵ����С��
�����ֵ����С��
C��c(H+)��c(OH-)=KW���¶Ȳ��䣬KW���䣻
D��ϡ�����У�OH-�����ʵ������
�ʴ�Ϊ��B��
��4��������ˮ�ķ�ӦΪ NH3+H2O![]() NH4++OH-��������һ����ɫ��ȼ��Һ�壬����ˮ�Լ��ԣ�����ˮ�е���ԭ���백���ƣ���N2H4+H2O
NH4++OH-��������һ����ɫ��ȼ��Һ�壬����ˮ�Լ��ԣ�����ˮ�е���ԭ���백���ƣ���N2H4+H2O![]() NH2NH3++OH-����N2H4+H2O
NH2NH3++OH-����N2H4+H2O![]() N2H5++OH-������һ����ӦҲ����ΪNH2NH3++H2O
N2H5++OH-������һ����ӦҲ����ΪNH2NH3++H2O![]() NH3N32++OH-����NH2NH3++H2O
NH3N32++OH-����NH2NH3++H2O![]() N2H62++OH-��N2H5++H2O
N2H62++OH-��N2H5++H2O![]() N2H62++OH-����
N2H62++OH-����
�ʴ�Ϊ��N2H4+H2O![]() NH2NH3++OH-����N2H4+H2O
NH2NH3++OH-����N2H4+H2O![]() N2H5++OH-����NH2NH3++H2O
N2H5++OH-����NH2NH3++H2O![]() NH3NH32++OH-����NH2NH3++H2O
NH3NH32++OH-����NH2NH3++H2O![]() N2H62++OH-��N2H5++H2O
N2H62++OH-��N2H5++H2O![]() N2H62++OH-����
N2H62++OH-����
��5���ɵ���ƽ�ⳣ����֪�����ԣ�H2B>CH3COOH>HB-������Na2B��Һ�м������CH3COOH��Һ�Ļ�ѧ����ʽΪ��CH3COOH+Na2B=CH3COONa+NaHB��
�ʴ�Ϊ��CH3COOH+Na2B=CH3COONa+NaHB��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�