��Ŀ����
����Ŀ����18�֣�2-����-3-�ȱ����ᣨF������Ҫ��ҽҩ�м��壬���Ʊ�����ͼ���£�
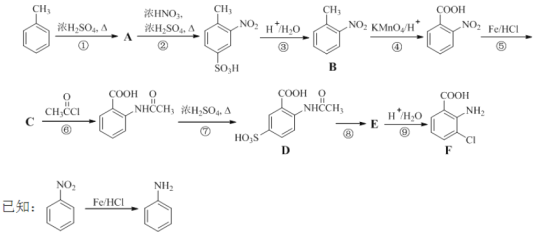
�ش��������⣺
��1��![]() �����в�ͬ��ѧ��������ԭ�ӹ���_______�֣�����ԭ����Ŀ���Ϊ_______��
�����в�ͬ��ѧ��������ԭ�ӹ���_______�֣�����ԭ����Ŀ���Ϊ_______��
��2��B������Ϊ_________��д��������������B������ͬ���칹��Ľṹ��ʽ_______��
a��������ֻ������ȡ�����һ�Ϊ��λ b�����ܷ���������Ӧ���ܷ���ˮ�ⷴӦ
��3��������δ���üױ�ֱ�������ķ����Ʊ�B�����Ǿ����٢ڢ�������Ӧ��ȡB����Ŀ����______________��
��4��д�����Ļ�ѧ��Ӧ����ʽ��_________���ò���Ӧ����ҪĿ����____________��
��5��д�����ķ�Ӧ�Լ���������_______________��F�к��������ŵ�����Ϊ__________��
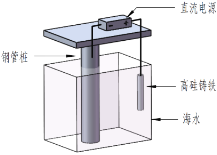
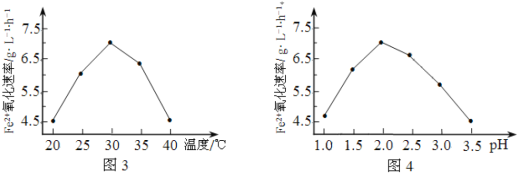
��6���ڷ�����д����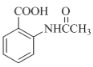 Ϊ��Ҫԭ�ϣ������ٲ����Ʊ����ļ��ۺ�������̡�
Ϊ��Ҫԭ�ϣ������ٲ����Ʊ����ļ��ۺ�������̡�
|
���𰸡���18�֣�
��1��4 13
��2��2-�����ױ����������ױ�
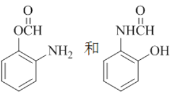
��3�����ⱽ���ϼ���λ����ԭ�ӱ�����ȡ��������ٸ������ռλ��
��4��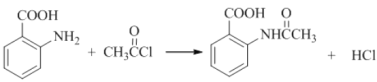 ��������
��������
��5��Cl2/FeCl3����Cl2/Fe�� �Ȼ�
��6��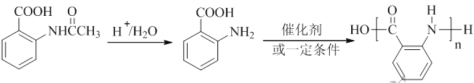
��������
��1���ױ������в�ͬ��ѧ��������ԭ�ӹ���4�֣�����ԭ����Ŀ���Ϊ13����
��2��B������Ϊ�������ױ�����2-�����ױ�����
B��ͬ���칹�����ܷ���������Ӧ����ȩ�����������ṹ�����ܷ���ˮ�ⷴӦ��˵�����������ṹ�����ļ��ṹ���ɴ˿ɵ�B��ͬ���칹���У�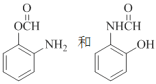 ��
��
��3�����ױ�ֱ�����������ø�����϶࣬�������ڼ��Ķ�λ����������Ӧ�����������������ױ��ȡ�
��4����Ӧ��Ϊ�ڰ����������������ȷ���ȡ����Ӧ������ʽΪ��
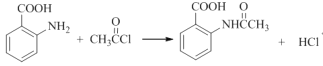 �����F�Ľṹ��֪���ò����Ŀ���DZ�����������ֹ������������
�����F�Ľṹ��֪���ò����Ŀ���DZ�����������ֹ������������
��5�����D��F�Ľṹ��֪����Ӧ������Clԭ�ӣ�����������Clԭ�ӵķ�������Fe����FeCl3����������������Cl2����ȡ����Ӧ��F�еĺ���������Ϊ�Ȼ���
��6�����ݲ����������۷�Ӧ�ɵ��������£�
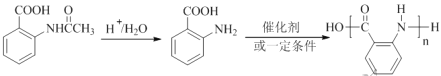 ��
��

����Ŀ����17�֣��±���Ԫ�����ڱ���һ����, ��Ա��е��١�����Ԫ��,��д���пհ�(��д��Ų��÷�):
���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0�� |
2 | �� | �� | �� | |||||
3 | �� | �� | �� | �� | �� | |||
4 | �� | �� |
��1������ЩԪ����,��ѧ��������õ���:_____������Ԫ�ط�����
��2��������������ˮ�����У�������ǿ�Ļ��������ѧʽ��/span>______��������ǿ�Ļ��������ѧʽ��:__________��
��3���ȽϢ���ݵ�����������Ӧ��ˮ���_________������ǿ������ѧʽ������ͨ��________________________________˵����д��Ӧ����ѧ����ʽ����
��4��ʵ������ȡ�ڵ��⻯�����ѧ����ʽ_______________________________ ���ڵ��⻯����ڵ�����������ˮ���ﷴӦ���õIJ�����ѧʽΪ______
��5���������γɶ������������һ���Ǻ���ɫ���壬���÷���ʽ˵�������岻�˲�����ˮ���ռ���ԭ��________________������ѧ����ʽ��ʾ��
��6���ȽϢ�����⻯�_________ ���ȶ�������ѧʽ��
��7��д���ܵĵ�����ˮ��Ӧ�����ӷ���ʽ_______________________��
��8��д����Ԫ�ص������ṹʾ��ͼ______,�����Ӱ뾶_________ S2-�������������д����Ԫ�������ڱ���λ��_______________________