��Ŀ����
ij�����ķ�Һ�к��д�����K+��Cl����Br��������������Ca2+��Mg2+��SO42����ij�о���ѧϰС���������ַ�Һ����ȡ�ϴ������Ȼ��ؾ��弰Һ�壨Br2��������������������̣�
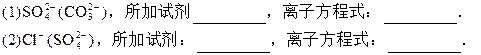
�ɹ��Լ�a��b��ѡ���Լ�������Na2CO3��Һ������K2CO3��Һ��KO H��Һ��BaCl2��Һ��Ba(NO3)2��Һ��H2O2��KMnO4(H+)��Һ��ϡHNO3��
H��Һ��BaCl2��Һ��Ba(NO3)2��Һ��H2O2��KMnO4(H+)��Һ��ϡHNO3��
������������̣��ش�������⣺
���Լ�aӦ��ѡ��_______________��
�Ʋ����١��ڡ��ۡ��ܡ��ݵ�������_________(����ĸ)��
������ȥ��ɫҺ��I�е�Ca2+��Mg2+��SO42-���ӣ�ѡ��b���������Լ������μ�˳�������� ���ѧʽ����
�ȵ���pH �������� ������������ ��
�������� ������������ ��
�ɲ��������õ��Ĵ������������� ��

�ɹ��Լ�a��b��ѡ���Լ�������Na2CO3��Һ������K2CO3��Һ��KO
 H��Һ��BaCl2��Һ��Ba(NO3)2��Һ��H2O2��KMnO4(H+)��Һ��ϡHNO3��
H��Һ��BaCl2��Һ��Ba(NO3)2��Һ��H2O2��KMnO4(H+)��Һ��ϡHNO3��������������̣��ش�������⣺
���Լ�aӦ��ѡ��_______________��
�Ʋ����١��ڡ��ۡ��ܡ��ݵ�������_________(����ĸ)��
| A����ȡ�����ˡ���Һ�����ˡ������ᾧ | B����ȡ����Һ�������ˡ������ᾧ |
| C����Һ����ȡ�����ˡ����ˡ������ᾧ | D����ȡ����Һ����Һ�����ˡ������ᾧ |
�ȵ���pH
 �������� ������������ ��
�������� ������������ ���ɲ��������õ��Ĵ������������� ��
��12�֣�
��1��H2O2��2�֣�
��2��B��2�֣�
��3��BaCl2��KOH��K2CO3��ֻҪK2CO3��BaCl2֮�ɣ���2�֣�
��4����ȥ��Һ�й�����CO32-��2�֣���ȡ������PH��ֽ���ڲ���Ƭ�ϣ���պ�д�����Һ�IJ������������ֽ���в�������ֽ��ɫ���������ɫ���Ƚ���ȷ����Һ��pH��3�֣�
��5��������1�֣�
��1��H2O2��2�֣�
��2��B��2�֣�
��3��BaCl2��KOH��K2CO3��ֻҪK2CO3��BaCl2֮�ɣ���2�֣�
��4����ȥ��Һ�й�����CO32-��2�֣���ȡ������PH��ֽ���ڲ���Ƭ�ϣ���պ�д�����Һ�IJ������������ֽ���в�������ֽ��ɫ���������ɫ���Ƚ���ȷ����Һ��pH��3�֣�
��5��������1�֣�
��

��ϰ��ϵ�д�
�����Ŀ
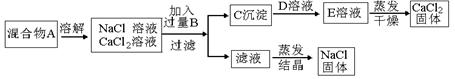
 �ӷ���ʽ�� ��
�ӷ���ʽ�� �� �������NaCl��CaCl2�������ȣ��ɳ��������C����������(��Ϊm1)�ͻ����A������(��Ϊm2)��ȷ��
�������NaCl��CaCl2�������ȣ��ɳ��������C����������(��Ϊm1)�ͻ����A������(��Ϊm2)��ȷ�� ���������NaCl��CaCl2��������Ϊ (��m1��m2��ʾ)
���������NaCl��CaCl2��������Ϊ (��m1��m2��ʾ) ��
�� �ڽ���Ʒ��ˮ�ܽ⣬�Ƴɴ�����Һ�����������Һ�м��������ij���Լ������ˣ��ܳ���ϴ�Ӻ�С�ĺ�ɣ��õ���������A������Һ�ڽ���ijһ�����������������������õ���������B������ʵ���еõ���ij�ֹ��塣
�ڽ���Ʒ��ˮ�ܽ⣬�Ƴɴ�����Һ�����������Һ�м��������ij���Լ������ˣ��ܳ���ϴ�Ӻ�С�ĺ�ɣ��õ���������A������Һ�ڽ���ijһ�����������������������õ���������B������ʵ���еõ���ij�ֹ��塣