��Ŀ����
��10�֣���һ���Ӻ�ˮ�л�õĴ��Σ��Ѿ������������ᴿ������С������ijɷֽ���̽�����������ν�һ���ᴿ��
̽��һ����������л�����ʲô���ʣ�
���ݺ�ˮ�ijɷֺͳ����ᴿ��ʵ����������Ƹô��ο��ܻ����е�������CaCl2��MgCl2������ʵ����֤�����Ʋ⣺ȡ�����ܽ⣬��������NaOH��Һ��Ŀ���Ǽ����Ƿ���� ���ѧʽ����ͬ���������ټ�������Na2CO3��Һ��Ŀ���Ǽ����Ƿ���� ��ʵ��֤��������κ��е�������CaCl2 ��
��
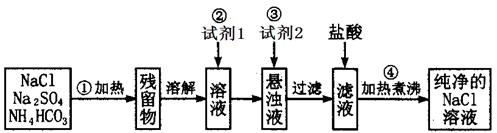
̽���������������NaCl�����������Ƕ��٣�
�����沽�����ʵ�飺�ٳ�ȡһ����������Ʒ�� �ڽ���Ʒ��ˮ�ܽ⣬�Ƴɴ�����Һ�����������Һ�м��������ij���Լ������ˣ��ܳ���ϴ�Ӻ�С�ĺ�ɣ��õ���������A������Һ�ڽ���ijһ�����������������������õ���������B������ʵ���еõ���ij�ֹ��塣
�ڽ���Ʒ��ˮ�ܽ⣬�Ƴɴ�����Һ�����������Һ�м��������ij���Լ������ˣ��ܳ���ϴ�Ӻ�С�ĺ�ɣ��õ���������A������Һ�ڽ���ijһ�����������������������õ���������B������ʵ���еõ���ij�ֹ��塣
��1���ڡ��ۡ��ݵIJ����У���ʹ�õ�ͬһ���� �������ƣ������ڲ���ں͢��еIJ� ��������ͬ����Ŀ�IJ�ͬ���ڲ�����е�Ŀ�� ���ڲ�����е�Ŀ���� ��
��������ͬ����Ŀ�IJ�ͬ���ڲ�����е�Ŀ�� ���ڲ�����е�Ŀ���� ��
��2��������м�����Լ��� ��д��ѧʽ����������н��еġ�ijһ�������� ��Ŀ���� ��
��3�������������Ϊ��Ҫ�����Ĺ���A ���ǹ���B�� ����A��B�����㲻ѡ����һ�ֹ���������� ��
���ǹ���B�� ����A��B�����㲻ѡ����һ�ֹ���������� ��
̽��һ����������л�����ʲô���ʣ�
���ݺ�ˮ�ijɷֺͳ����ᴿ��ʵ����������Ƹô��ο��ܻ����е�������CaCl2��MgCl2������ʵ����֤�����Ʋ⣺ȡ�����ܽ⣬��������NaOH��Һ��Ŀ���Ǽ����Ƿ���� ���ѧʽ����ͬ���������ټ�������Na2CO3��Һ��Ŀ���Ǽ����Ƿ���� ��ʵ��֤��������κ��е�������CaCl2
 ��
��̽���������������NaCl�����������Ƕ��٣�
�����沽�����ʵ�飺�ٳ�ȡһ����������Ʒ��
 �ڽ���Ʒ��ˮ�ܽ⣬�Ƴɴ�����Һ�����������Һ�м��������ij���Լ������ˣ��ܳ���ϴ�Ӻ�С�ĺ�ɣ��õ���������A������Һ�ڽ���ijһ�����������������������õ���������B������ʵ���еõ���ij�ֹ��塣
�ڽ���Ʒ��ˮ�ܽ⣬�Ƴɴ�����Һ�����������Һ�м��������ij���Լ������ˣ��ܳ���ϴ�Ӻ�С�ĺ�ɣ��õ���������A������Һ�ڽ���ijһ�����������������������õ���������B������ʵ���еõ���ij�ֹ��塣��1���ڡ��ۡ��ݵIJ����У���ʹ�õ�ͬһ���� �������ƣ������ڲ���ں͢��еIJ�
 ��������ͬ����Ŀ�IJ�ͬ���ڲ�����е�Ŀ�� ���ڲ�����е�Ŀ���� ��
��������ͬ����Ŀ�IJ�ͬ���ڲ�����е�Ŀ�� ���ڲ�����е�Ŀ���� ����2��������м�����Լ��� ��д��ѧʽ����������н��еġ�ijһ�������� ��Ŀ���� ��
��3�������������Ϊ��Ҫ�����Ĺ���A
 ���ǹ���B�� ����A��B�����㲻ѡ����һ�ֹ���������� ��
���ǹ���B�� ����A��B�����㲻ѡ����һ�ֹ���������� ��̽��һ�� MgCl2 �� CaCl2
̽��������1�� ������ �� �ӿ��ܽ� �� ��ֹҺ�ν���
��2��Na2CO3����ε���ϡ���ᣬֱ�����ٲ�������Ϊֹ�� �� ��ȥ����Na2CO3
��3�� A �� �������õ��Ȼ����У���һ������̼���Ƴ��Ȼ���ʱ���ɵģ�����һ�����ǹ�����̼���������ᷴӦ�õ��ģ��������ڼ���
̽��������1�� ������ �� �ӿ��ܽ� �� ��ֹҺ�ν���
��2��Na2CO3����ε���ϡ���ᣬֱ�����ٲ�������Ϊֹ�� �� ��ȥ����Na2CO3
��3�� A �� �������õ��Ȼ����У���һ������̼���Ƴ��Ȼ���ʱ���ɵģ�����һ�����ǹ�����̼���������ᷴӦ�õ��ģ��������ڼ���
��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
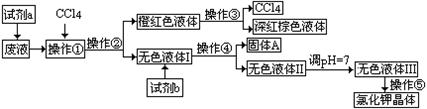
 H��Һ��BaCl2��Һ��Ba(NO3)2��Һ��H2O2��KMnO4(H+)��Һ��ϡHNO3��
H��Һ��BaCl2��Һ��Ba(NO3)2��Һ��H2O2��KMnO4(H+)��Һ��ϡHNO3�� �������� ������������ ��
�������� ������������ �� ������ȷ����
������ȷ����