��Ŀ����
����Һ�е����ӽ��м���������ʵ�����ý��۲��������ǣ� ��
| A������Һ�м���ϡ������ټ���AgNO3��Һ�а�ɫ�������ɣ�����Һ�п��ܺ���Cl- |
| B������Һ�м���ˣӣã���Һ���۲�������������������ˮ����Һ���ɫ��˵����Һ��һ�����У�e2+ |
| C������Һ�м����aOH��Һ�����Ⱥ����ʹʪ��ĺ�ɫʯ����ֽ���������壬����Ʋ����Һ�к���NH4+ |
| D������Һ�м������ᣬ����ʹ����ʯ��ˮ����ǵ���ɫ��ζ���壬���Ʋ���Һ�к���CO32-�� |
D
A��ȷ��Ϊ�����ӵļ��鷽����B��ȷ���������ӵļ��鷽����C��ȷ�������ӵļ��鷽����D��������Һ�м������ᣬ����ʹ����ʯ��ˮ����ǵ���ɫ��ζ���壬���Ʋ���Һ�к���CO32-��HCO3-��

��ϰ��ϵ�д�
 ����������ϵ�д�
����������ϵ�д� �Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�����Ŀ
 H��Һ��BaCl2��Һ��Ba(NO3)2��Һ��H2O2��KMnO4(H+)��Һ��ϡHNO3��
H��Һ��BaCl2��Һ��Ba(NO3)2��Һ��H2O2��KMnO4(H+)��Һ��ϡHNO3�� �������� ������������ ��
�������� ������������ ��
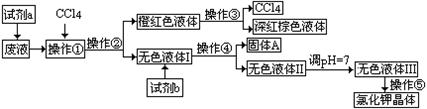
 ����ѧϰС������˽���MnO2�����н϶��MnO��MnCO3����Ʒת��Ϊ��MnO2��ʵ�飬���������£�
����ѧϰС������˽���MnO2�����н϶��MnO��MnCO3����Ʒת��Ϊ��MnO2��ʵ�飬���������£�
 ����Ӧ�����ӷ���ʽΪ��5Mn2+ + 2ClO3- + 4H2O �� 5MnO2�� + Cl2��
����Ӧ�����ӷ���ʽΪ��5Mn2+ + 2ClO3- + 4H2O �� 5MnO2�� + Cl2�� + 8H+
+ 8H+ ��
��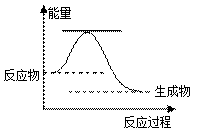
 ������ȷ����
������ȷ����