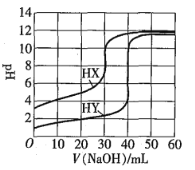
��Ŀ����
����Ŀ�����������ʵ���Һ��CH3COOH����HCl����H2SO4����NaHSO4
(1)��������Һ�����ʵ���Ũ����ͬ����c(H+)�Ĵ�С˳��Ϊ______(����ű�ʾ����ͬ)��
(2)��������Һ��c(H+)��ͬ�������ʵ���Ũ�ȵĴ�С˳��Ϊ________��
(3)��6 g CH3COOH����ˮ�Ƴ�1 L��Һ������Һ�����ʵ���Ũ��Ϊ________�����ⶨ��Һ��c(CH3COO-)Ϊ1.4��10��3 mol��L��1�����¶��´���ĵ��볣��Ka=________���¶����ߣ�Ka��______(�������������������������С������ͬ)����������CH3COONa��c(H��) _________��Ka________��ԭ����_____________��
���𰸡���>��=��>�� ��>��=��>�� 0.1mol/L 1.96��10-5 ��� ��С ���� Kwֻ���¶��йأ��¶Ȳ��䣬Kw����
��������
(1)������һԪ���ᡢ������һԪǿ�ᡢ�����Ƕ�Ԫǿ�ᡢ���������൱��һԪǿ�
(2)������һԪ���ᡢ������һԪǿ�ᡢ�����Ƕ�Ԫǿ�ᡢ���������൱��һԪǿ�ᣬ�ݴ˷�����
(3)�ȸ���n=![]() ����CH3COOH�����ʵ�����Ȼ�����c=
����CH3COOH�����ʵ�����Ȼ�����c=![]() �������ʵ���Ũ�ȣ�����k=
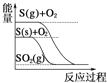
�������ʵ���Ũ�ȣ�����k=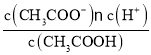 �������С���ж����µ���ƽ�ⳣ���ı仯��������������������ʵĵ���ƽ���Ӱ���ж�����Ũ�ȵı仯������ƽ�ⳣ��ֻ���¶�Ӱ��.
�������С���ж����µ���ƽ�ⳣ���ı仯��������������������ʵĵ���ƽ���Ӱ���ж�����Ũ�ȵı仯������ƽ�ⳣ��ֻ���¶�Ӱ��.
(1)������һԪ���ᡢ������һԪǿ�ᡢ�����Ƕ�Ԫǿ�ᡢ���������൱��һԪǿ�ᣬ����CH3COOH����HCl����H2SO4����NaHSO4��Һ�����ʵ���Ũ����ͬ����c(H+)�Ĵ�С˳��Ϊ����>��=��>�٣�
(2)������һԪ���ᡢ������һԪǿ�ᡢ�����Ƕ�Ԫǿ�ᡢ���������൱��һԪǿ�ᣬ����CH3COOH����HCl����H2SO4����NaHSO4��Һ��c(H+)��ͬ���������ʵ���Ũ�ȴ�С˳��Ϊ����>��=��>�ۣ�
(3)n(CH3COOH)=![]() =0.1mol���������Һ�����ʵ���Ũ��c(CH3COOH)=
=0.1mol���������Һ�����ʵ���Ũ��c(CH3COOH)=![]() =0.1mol/L�����ݴ���ĵ���ƽ�⣺CH3COOH
=0.1mol/L�����ݴ���ĵ���ƽ�⣺CH3COOH![]() CH3COO-+H+��֪����Һ��c(H+)=c(CH3COO-)=1.4��10-3mol/L�������������ʵĵ���ƽ�ⳣ�������֪K=
CH3COO-+H+��֪����Һ��c(H+)=c(CH3COO-)=1.4��10-3mol/L�������������ʵĵ���ƽ�ⳣ�������֪K=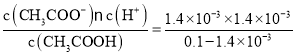 =1.96��10-5��
=1.96��10-5��
�������������������������¶ȴٽ�����ĵ��룬ʹ����ƽ�ⳣ�������������Һ�м�������ƣ����������Ũ��������ĵ���ƽ�������ƶ�����Һ��������Ũ�ȼ�С�����ڵ���ƽ�ⳣ��ֻ���¶��йأ��¶Ȳ��䣬����ĵ���ƽ�����Ͳ��䡣