��Ŀ����
��10�֣�������ѧ��ѧ��Ӧԭ��������������⣺
��1��ij��Һ��������ͬ���ʵ��������ʣ���Һ��ֻ����OH����H����NH4����Cl���������ӣ���c��NH4������c��Cl������c��OH������c��H�������������������� _________ ��
��2��0.1 mol��L-1�İ�ˮ��0.05 mol��L-1��ϡ����������ϣ������ӷ���ʽ��ʾ��Ϻ���Һ������ԣ� ��
��3����ʯī�缫���100 mL 0.1 mol��L-1CuSO4��Һ���������ϲ�����������ʵ���Ϊ0.01 mol��������������Cu������Ϊ g��
��4����AgCl�ֱ�����5mL H2O ��10mL0.2mol��L-1 MgCl2��20 mL0.5mol��L-1 NaCl��Һ��40 mL0.1mol��L-1HCl���ܽ������ͣ�����Һ��Ag+��Ũ���ɴ�С��˳����________________��
��5����20 mL���������Ļ��Һ����μ���pH��13��Ba(OH)2��Һ������BaSO4��������ͼ��ʾ��B����Һ��pH��7��������Һ����仯������c��HCl����_________mol��L-1��
��1��ij��Һ��������ͬ���ʵ��������ʣ���Һ��ֻ����OH����H����NH4����Cl���������ӣ���c��NH4������c��Cl������c��OH������c��H�������������������� _________ ��
��2��0.1 mol��L-1�İ�ˮ��0.05 mol��L-1��ϡ����������ϣ������ӷ���ʽ��ʾ��Ϻ���Һ������ԣ� ��
��3����ʯī�缫���100 mL 0.1 mol��L-1CuSO4��Һ���������ϲ�����������ʵ���Ϊ0.01 mol��������������Cu������Ϊ g��
��4����AgCl�ֱ�����5mL H2O ��10mL0.2mol��L-1 MgCl2��20 mL0.5mol��L-1 NaCl��Һ��40 mL0.1mol��L-1HCl���ܽ������ͣ�����Һ��Ag+��Ũ���ɴ�С��˳����________________��
��5����20 mL���������Ļ��Һ����μ���pH��13��Ba(OH)2��Һ������BaSO4��������ͼ��ʾ��B����Һ��pH��7��������Һ����仯������c��HCl����_________mol��L-1��

��1��NH4Cl�� NH3��H2O (2��) ��2��NH4++ H2O NH3?H2O + H+ (2��)
NH3?H2O + H+ (2��)
��3��0.64 (2��) ��4���� > �� > ��> �� (2��) ��5��0.2 (2��)
 NH3?H2O + H+ (2��)
NH3?H2O + H+ (2��)��3��0.64 (2��) ��4���� > �� > ��> �� (2��) ��5��0.2 (2��)
��1��c��NH4������c��Cl������c��OH������c��H������������Һ�Լ��ԣ����������NH4Cl�� NH3��H2O��
��2��0.1 mol��L-1�İ�ˮ��0.05 mol��L-1��ϡ����������ϣ�����ǡ�÷�Ӧ����������泥�ˮ�������ԣ����Է���ʽΪNH4++ H2O NH3?H2O + H+��
NH3?H2O + H+��
��3�����Ե缫�������ͭ������������������������ͭ�����ݵ��ӵ�ʧ�غ��֪��ͭ�����ʵ�����0.01mol��4��2��0.01mol����������0.01mol��64g/mol��0.64g��
��4�������ܶȻ���������ʽ��֪����Һ��������Ũ��Խ��������Ũ�Ⱦ�ԽС�����Ը���Һ��Ag+��Ũ���ɴ�С��˳���Ǣ� > �� > ��> �ۡ�
��5������ͼ���֪�������ᷴӦ������������Һ�����20mol�������ᷴӦ������������Һ�����40ml�����������Ũ����0.1mol/L��0.04L��0.02L��0.2mol/L��
��2��0.1 mol��L-1�İ�ˮ��0.05 mol��L-1��ϡ����������ϣ�����ǡ�÷�Ӧ����������泥�ˮ�������ԣ����Է���ʽΪNH4++ H2O
 NH3?H2O + H+��
NH3?H2O + H+����3�����Ե缫�������ͭ������������������������ͭ�����ݵ��ӵ�ʧ�غ��֪��ͭ�����ʵ�����0.01mol��4��2��0.01mol����������0.01mol��64g/mol��0.64g��
��4�������ܶȻ���������ʽ��֪����Һ��������Ũ��Խ��������Ũ�Ⱦ�ԽС�����Ը���Һ��Ag+��Ũ���ɴ�С��˳���Ǣ� > �� > ��> �ۡ�
��5������ͼ���֪�������ᷴӦ������������Һ�����20mol�������ᷴӦ������������Һ�����40ml�����������Ũ����0.1mol/L��0.04L��0.02L��0.2mol/L��

��ϰ��ϵ�д�
�����Ŀ
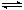 CH3OH(g)+H2O(g)��ijЩ��ѧ���ļ����������±���
CH3OH(g)+H2O(g)��ijЩ��ѧ���ļ����������±���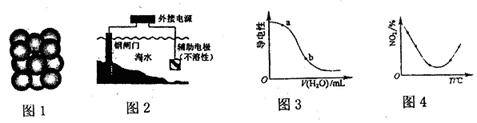
 N204(g)����ͬʱ�����N02���������ߣ���÷�Ӧ�ġ�H<O
N204(g)����ͬʱ�����N02���������ߣ���÷�Ӧ�ġ�H<O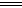 N2(g)+2H2O(l) ��H="-a" kJ/mol
N2(g)+2H2O(l) ��H="-a" kJ/mol Cu2O+H2������������ӦʽΪ�� ��
Cu2O+H2������������ӦʽΪ�� �� 2H2(g)��O2(g) ��H����484 kJ��mol��1
2H2(g)��O2(g) ��H����484 kJ��mol��1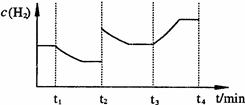
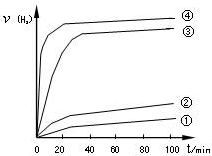
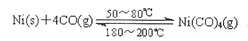

 ��3����ҵ����һ����̼��ȡ�����ķ�ӦΪ��CO��g��+H2O CO2(g)+H2��g������֪420��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��Ϊ9.0�������Ӧ��ʼʱ����2L���ܱ������г���CO��H2O�����ʵ�������0.60mol,5minĩ�ﵽƽ�⣬���ʱCO��ת����Ϊ ��H2��ƽ����������Ϊ ��
��3����ҵ����һ����̼��ȡ�����ķ�ӦΪ��CO��g��+H2O CO2(g)+H2��g������֪420��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��Ϊ9.0�������Ӧ��ʼʱ����2L���ܱ������г���CO��H2O�����ʵ�������0.60mol,5minĩ�ﵽƽ�⣬���ʱCO��ת����Ϊ ��H2��ƽ����������Ϊ ��
 CH3OH(g) ��H��
CH3OH(g) ��H��