��Ŀ����
����Ŀ������һ��ǿ��ԭ������NaClO��NH3��Ӧ�����������£�N2H4�����䷴Ӧ�Ļ�ѧ����ʽΪ��NaClO+2NH3= N2H4+NaCl+H2O��
������1000 g��������Ϊ25.6%������Һ������Ҫ___________L����״����NH3��
����ҵ����������Һ�к��������ƻ�Ӱ�������µIJ�Ʒ�������ⶨ����������Ʒ�е������ƺ����ķ������£�ȡ10.00 mL ����NaClO��Һ�������������H2O2��������������ȫ��ԭ��ClO3-�����������¾���ǿ�����ԣ������������¼����������ԣ���������У���ȴ�����£��������������ԣ��ټ���0.1000 mol��L-1 ������������Һ30.00 mL����ַ�Ӧ����0.01000 mol��L-1����K2Cr2O7��Һ�ζ����յ㣨Cr2O72-����ԭΪCr3+�������ĸ���Һ20.00 mL��
����H2O2��������Ʒ�Ӧ�����ӷ���ʽΪ___________��
��ʵ���м�����е�Ŀ����___________��
��������Ʒ��NaClO3�ĺ�������g��L-1��ʾ����д��������̡�___________
���𰸡� 358.4 H2O2+ ClO- �� Cl-+ O2��+ H2O ��ȥ������H2O2 n(K2Cr2O7)= 0.01000 mol��L-1��20.00��10-3 L=2��10-4 mol
����Cr2O72-+6Fe2++14H+��2Cr3++6Fe3++7H2O
��K2Cr2O7��Ӧ��Fe2+��n(Fe2+)1= 2��10-4 mol��6=1.2��10-3 mol
��ClO3-��Ӧ��Fe2+��n(Fe2+)2=0.1000 mol��L-1��30.00��10-3 L��1.2��10-3 mol=1.8��10-3 mol
����ClO3-+6Fe2++6H+��Cl-+6Fe3++3H2O
��10 mLNaClO��Һ�����У�n(NaClO3)= 1.8��10-3 mol ��![]() =3��10-4 mol
=3��10-4 mol
��Ʒ��NaClO3�ĺ���=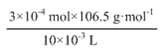 =3.195 g��L-1
=3.195 g��L-1
����������1000 g��������Ϊ25.6%������Һ���µ����ʵ���Ϊ![]() =8mol����μӷ�Ӧ��NH3�����ʵ���Ϊ8mol��2=16mol������µ����Ϊ16mol��22.4L/mol=358.4L��
=8mol����μӷ�Ӧ��NH3�����ʵ���Ϊ8mol��2=16mol������µ����Ϊ16mol��22.4L/mol=358.4L��
�Ƣ���H2O2���������Ʊ�����Ϊ������ͬʱ�õ�NaCl��������Ӧ�����ӷ���ʽΪH2O2+ ClO- �� Cl-+ O2��+ H2O��
��H2O2���ȶ��������ֽ⣬ͨ��������г�ȥ������H2O2 ��
��n(K2Cr2O7)= 0.01000 mol��L-1��20.00��10-3 L=2��10-4 mol
����Cr2O72-+6Fe2++14H+��2Cr3++6Fe3++7H2O
��K2Cr2O7��Ӧ��Fe2+��n(Fe2+)1= 2��10-4 mol��6=1.2��10-3 mol
��ClO3-��Ӧ��Fe2+��n(Fe2+)2=0.1000 mol��L-1��30.00��10-3 L��1.2��10-3 mol=1.8��10-3 mol
����ClO3-+6Fe2++6H+��Cl-+6Fe3++3H2O
��10 mLNaClO��Һ�����У�n(NaClO3)= 1.8��10-3 mol ��![]() =3��10-4 mol
=3��10-4 mol
��Ʒ��NaClO3�ĺ���= =3.195 g��L-1��
=3.195 g��L-1��