��Ŀ����
����Ŀ������������ȷ���ǣ� ��
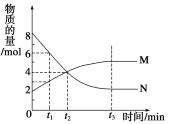
A.0.1mol��L��1��ˮ�У�c(OH��)=c(NH4+)
B.10 mL 0.02mol��L��1HCl��Һ��10 mL 0.02mol��L��1Ba(OH)2��Һ��ֻ��,����Ϻ���Һ�����Ϊ20 mL������Һ��pH=11
C.��0.1mol��L��1CH3COONa��Һ�У�c(OH��)=c(CH3COOH)��c(H+)
D.0.1mol��L��1ij��Ԫ����ǿ����NaHA��Һ�У�c(Na��)=2c(A2��)��c(HA��)��c(H2A)
���𰸡�C
��������A���ɵ���غ��֪��ˮ��Һ�д���c��OH-��=c��NH4+��+c��H+������c��OH-����c��NH4+������A���������⣻B��c��OH-��= ![]() =0.01mol/L����pH=12����B���������⣻
=0.01mol/L����pH=12����B���������⣻
C��CH3COONa��Һ�д��������غ㣬Ϊc��OH-��=c��CH3COOH��+c��H+������C�������⣻
D��NaHA��Һ�д��������غ㣬Ϊc��Na+��=c��A2-��+c��HA-��+c��H2A������D���������⡣
���Դ��ǣ�C

��ϰ��ϵ�д�
 �����ÿ�ʱѵ��ϵ�д�
�����ÿ�ʱѵ��ϵ�д� ��Ԫȫ��������ϵ�д�
��Ԫȫ��������ϵ�д� �»ƸԱ����ܾ�ϵ�д�
�»ƸԱ����ܾ�ϵ�д�
�����Ŀ