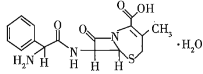
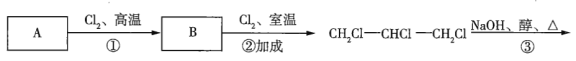
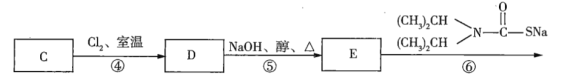
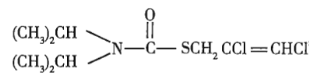��Ŀ����
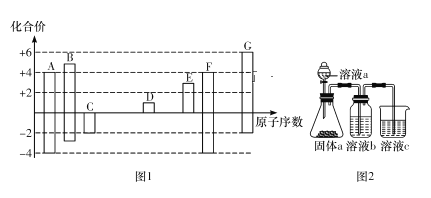
����Ŀ��ͼ1�Dz��ֶ�����Ԫ�صij������ϼ���ԭ�������Ĺ�ϵͼ��
��ش��������⣺
(1)Ԫ��F�����ڱ��е�λ��Ϊ________________
(2)C��D��E��G�ļ����Ӱ뾶�ɴ�С��˳��Ϊ_______________(�����ӷ��ű�ʾ)��
(3)��Ԫ������X�Ǻ���Ԫ��A��18���ӷ��ӣ�3 g X(g)��25 �� 101 kPa ����ȫȼ�������ȶ��Ļ�����ʱ�ų�Q kJ��������д����ʾXȼ���ȵ��Ȼ�ѧ����ʽ��________________
(4)ijͬѧ���ʵ����ͼ2��ʾװ��֤��Ԫ��A��B��F�ķǽ�����ǿ��(������Һb����Һc������)��
����ҺbΪ_________________
����Һc�з�����Ӧ�����ӷ���ʽΪ__________________
���𰸡��������ڵڢ�A�� S2��>O2��>Na��>Al3�� C2H6(g)��7/2O2(g)===2CO2(g)��3H2O(l) ��H����10QkJ/mol ����NaHCO3��Һ SiO32-��CO2��H2O===H2SiO3����CO32-
��������
���������֪�����⿼��Ԫ�����ڱ���Ԫ�ػ��ϼۡ����Ӱ뾶��С���Ȼ�ѧ����ʽ����д������Ԫ�������ɡ����Ӱ뾶��С�ȽϷ������Ȼ�ѧ����ʽ��д���������
��1����ͼ1�����ɵã�AΪC��BΪN��CΪO��DΪNa��EΪAl��FΪSi��GΪS,���F�����ڱ��е�λ��Ϊ�������ڵ���A�壻
�ʴ�Ϊ���������ڵ���A�壻
��2�����Ӳ�Խ�࣬���Ӱ��Խ������ͬ�����Ų���������ԭ������������Ӱ뾶С����S2��>O2��>Na��>Al3����
�ʴ�Ϊ��S2��>O2��>Na��>Al3����
(3) ��Ԫ������X�Ǻ���Ԫ��A��18���ӷ��ӣ�XΪC2H6��3 g X(g)��25 �� 101 kPa ����ȫȼ�������ȶ��Ļ�����ʱ�ų�Q kJ����������Xȼ���ȵ��Ȼ�ѧ����ʽΪC2H6(g)��![]() O2(g)===2CO2(g)��3H2O(l) ��H����10QkJ/mol��
O2(g)===2CO2(g)��3H2O(l) ��H����10QkJ/mol��
�ʴ�Ϊ��C2H6(g)��![]() O2(g)===2CO2(g)��3H2O(l) ��H����10QkJ/mol��
O2(g)===2CO2(g)��3H2O(l) ��H����10QkJ/mol��
��4����֤��Ԫ��A��B��F�ķǽ�����ǿ������Ӧ��A��B��F��Ӧ������������ˮ���������Ӧ�ν��з�Ӧ����֤�������Һa��b��c�ֱ�ΪHNO3������NaHCO3��Na2SiO3��Һ��
�ʴ�Ϊ������NaHCO3��Һ��
��Һb�в����Ķ�����̼ͨ��c�� Na2SiO3��Һ�з�����Ӧ�����ӷ���ʽΪSiO32-��CO2��H2O===H2SiO3����CO32-��
�ʴ�Ϊ��SiO32-��CO2��H2O===H2SiO3����CO32-��

����Ŀ����![]() ���������������Ϣ�����ʾ��
���������������Ϣ�����ʾ��
�� | �����Ϣ |
| ��ȫȼ�յIJ�����n(CO2):n(H2O)=2:1��28<Mr(A)<60������ʹ������Ȼ�̼��Һ��ɫ��һ�ȴ���ֻ��һ�ֽṹ |
| �������������³�ѹ�³���̬��������������� |
(1)��![]() ��ʵ��ʽ��______________��
��ʵ��ʽ��______________��
(2)��![]() �Ľṹ��ʽ��______________��
�Ľṹ��ʽ��______________��
(3)��![]() �����ֶ������Ľṹ��ʽ�ֱ�Ϊ___________��___________��___________��
�����ֶ������Ľṹ��ʽ�ֱ�Ϊ___________��___________��___________��