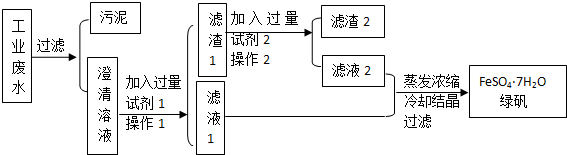
��Ŀ����
ijǿ������ҺX�п��ܺ���NH4+��Fe2+��Al3+��CO32-��SO42-��Cl-��NO3-�е������֣�ij�о���ѧϰС��Ϊ̽����ҺX����ɣ���������ʵ�飨����YΪ��ɫ��ζ�����壩��
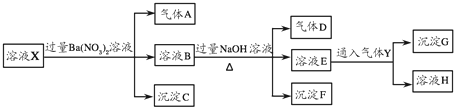
�ش��������⣺
��1����ҺX�п϶����ڵ������� ��
��2��д����������A�����ӷ���ʽ ��
��3��д������ҺE��ͨ������Yʱһ�����������ӷ���ʽ ��
��4������Һ�п��ܴ��ڵ����� ��
��5���ɳ���F��KOH��Һ����KClO��Ӧ���Ƶ�һ�����͡���Ч�����ˮ����������֪��ˮ��������һ�ֺ������Σ�ȡ3.96g��������ˮ���μ�����ϡ������ټ���0.04mol���ۣ�ǡ����ȫ��Ӧ����Fe2+����Ӧ�����Һ����һ������KOH��Һ���պý�Fe2+������ȫ�����ˣ���������ּ��Ⱥ�õ�0.03mol Fe2O3������Һ��һ�������������ɵõ�һ�ִ����IJ����ᾧˮ�ĺ���������Σ����Ǹ��Σ�Z����������ʵ���Ϊ0.08mol��
��Z�Ļ�ѧʽΪ ��ˮ��������Ħ������Ϊ ��
����ɲ���ƽ���з���ʽ�� + KClO+ KOH�� + KCl+ H2O��

�ش��������⣺
��1����ҺX�п϶����ڵ�������
��2��д����������A�����ӷ���ʽ
��3��д������ҺE��ͨ������Yʱһ�����������ӷ���ʽ
��4������Һ�п��ܴ��ڵ�����
��5���ɳ���F��KOH��Һ����KClO��Ӧ���Ƶ�һ�����͡���Ч�����ˮ����������֪��ˮ��������һ�ֺ������Σ�ȡ3.96g��������ˮ���μ�����ϡ������ټ���0.04mol���ۣ�ǡ����ȫ��Ӧ����Fe2+����Ӧ�����Һ����һ������KOH��Һ���պý�Fe2+������ȫ�����ˣ���������ּ��Ⱥ�õ�0.03mol Fe2O3������Һ��һ�������������ɵõ�һ�ִ����IJ����ᾧˮ�ĺ���������Σ����Ǹ��Σ�Z����������ʵ���Ϊ0.08mol��
��Z�Ļ�ѧʽΪ
����ɲ���ƽ���з���ʽ��
���㣺������ƶ�,������ԭ��Ӧ����ʽ����ƽ,���ʵļ���ͼ����ʵ�鷽�����
ר�⣺���ʼ��������
��������ǿ������Һ��һ���������CO32-����ҺX�м���������ᱵ���ɳ�������ó���ΪBaSO4������˵����Һ�к���SO42-���ӣ���������A��AӦΪNO��˵����Һ�к��л�ԭ�����ӣ�һ��ΪFe2+���ӣ�����Һ�в��ܴ���NO3-����ҺB�м������NaOH��Һ����������D����DΪNH3��˵����Һ�к���NH4+���ӣ�����FΪFe��OH��3����ҺE��ͨ��Y��CO2���壩���ɳ���G����G��һ����̼�ᱵ�����ܺ�Al��OH��3����E����ΪNaAlO2����ҺHΪNaHCO3������˵����Һ�к���������ΪNH4+��Fe2+��һ����������ΪSO42-��һ������CO32-��NO3-���ӣ�����ȷ���Ƿ���Al3+��Cl-���Դ������
���
�⣺��ǿ������Һ��һ���������CO32-����ҺX�м���������ᱵ���ɳ�������ó���ΪBaSO4������˵����Һ�к���SO42-���ӣ���������A��AӦΪNO��˵����Һ�к��л�ԭ�����ӣ�һ��ΪFe2+���ӣ�����Һ�в��ܴ���NO3-����ҺB�м������NaOH��Һ����������D����DΪNH3��˵����Һ�к���NH4+���ӣ�����FΪFe��OH��3����ҺE��ͨ��Y��CO2���壩���ɳ���G����G��һ����̼�ᱵ��G���ܺ�Al��OH��3����E����ΪNaAlO2����ҺHΪNaHCO3������˵����Һ�к���������ΪNH4+��Fe2+��һ����������ΪSO42-��һ������CO32-��NO3-���ӣ�����ȷ���Ƿ���Al3+��Cl-��
��1��������������֪��X�к�����ΪNH4+��Fe2+��SO42-���ʴ�Ϊ��NH4+��Fe2+��SO42-��
��2����������A�����ӷ���ʽΪ3Fe2++4H++NO3-�T3Fe3++NO��+2H2O���ʴ�Ϊ��3Fe2++4H++NO3-�T3Fe3++NO��+2H2O��
��3������ҺE��ͨ������Yʱһ�����������ӷ���ʽBa2++2OH-+CO2�TBaCO3��+H2O���ʴ�Ϊ��Ba2++2OH-+CO2�TBaCO3��+H2O��
��4��������������֪����Һ�п��ܴ��ڵ�����ΪCl-��Al3+���ʴ�Ϊ��Cl-��Al3+��
��5������FΪFe��OH��3��������ּ��Ⱥ�õ�0.03mol Fe2O3��n��Fe��=0.06mol���μ�����ϡ������ټ���0.04mol���ۣ�ǡ����ȫ��Ӧ����Fe2+�������к�n��Fe��=0.02mol�������ɵõ�һ�ִ����IJ����ᾧˮ�ĺ���������Σ����Ǹ��Σ�Z����Ԫ���غ��֪��ZΪK2SO4����������ʵ���Ϊ0.08mol����K+�����ʵ���Ϊ0.16mol����Ӧ�����Һ����һ������KOH��Һ���պý�Fe2+������ȫ�������ĵ�KOHΪ0.06mol��2=0.12mol����ˮ��������K+�����ʵ���Ϊ0.16mol-0.12mol=0.04mol��ˮ��������һ�ֺ������Σ�����F��KClO�������ɣ���+6��Fe�������������ӦΪFeO42-������ΪK2FeO4�������ʵ���Ϊ
=0.02mol��
��������������֪��ZΪK2SO4��ˮ��������Ħ������Ϊ
=198g/mol���ʴ�Ϊ��K2SO4��198 g/mol��
��Fe��OH��3���л�ԭ�ԣ�KClO���������ԣ�FeԪ�صĻ��ϼ���+3������Ϊ+6�ۣ�ClԪ�صĻ��ϼ���+1�۽���Ϊ-1�ۣ���С������Ϊ6���ɵ����غ��ԭ���غ��֪���÷�ӦΪ2Fe��OH��3+3KClO+4KOH�T2K2FeO4+3KCl+5H2O���ʴ�Ϊ��2��Fe��OH��3��3��4��2��K2FeO4��3��5��
��1��������������֪��X�к�����ΪNH4+��Fe2+��SO42-���ʴ�Ϊ��NH4+��Fe2+��SO42-��
��2����������A�����ӷ���ʽΪ3Fe2++4H++NO3-�T3Fe3++NO��+2H2O���ʴ�Ϊ��3Fe2++4H++NO3-�T3Fe3++NO��+2H2O��
��3������ҺE��ͨ������Yʱһ�����������ӷ���ʽBa2++2OH-+CO2�TBaCO3��+H2O���ʴ�Ϊ��Ba2++2OH-+CO2�TBaCO3��+H2O��
��4��������������֪����Һ�п��ܴ��ڵ�����ΪCl-��Al3+���ʴ�Ϊ��Cl-��Al3+��
��5������FΪFe��OH��3��������ּ��Ⱥ�õ�0.03mol Fe2O3��n��Fe��=0.06mol���μ�����ϡ������ټ���0.04mol���ۣ�ǡ����ȫ��Ӧ����Fe2+�������к�n��Fe��=0.02mol�������ɵõ�һ�ִ����IJ����ᾧˮ�ĺ���������Σ����Ǹ��Σ�Z����Ԫ���غ��֪��ZΪK2SO4����������ʵ���Ϊ0.08mol����K+�����ʵ���Ϊ0.16mol����Ӧ�����Һ����һ������KOH��Һ���պý�Fe2+������ȫ�������ĵ�KOHΪ0.06mol��2=0.12mol����ˮ��������K+�����ʵ���Ϊ0.16mol-0.12mol=0.04mol��ˮ��������һ�ֺ������Σ�����F��KClO�������ɣ���+6��Fe�������������ӦΪFeO42-������ΪK2FeO4�������ʵ���Ϊ
| 0.04mol |
| 2 |
��������������֪��ZΪK2SO4��ˮ��������Ħ������Ϊ
| 3.96g |
| 0.02mol |
��Fe��OH��3���л�ԭ�ԣ�KClO���������ԣ�FeԪ�صĻ��ϼ���+3������Ϊ+6�ۣ�ClԪ�صĻ��ϼ���+1�۽���Ϊ-1�ۣ���С������Ϊ6���ɵ����غ��ԭ���غ��֪���÷�ӦΪ2Fe��OH��3+3KClO+4KOH�T2K2FeO4+3KCl+5H2O���ʴ�Ϊ��2��Fe��OH��3��3��4��2��K2FeO4��3��5��
���������⿼��������ƶϣ�Ϊ��Ƶ���㣬�������ӵķ�Ӧ���غ㷨�ƶ�X�е����Ӽ����ü����ƶϣ�5���е�ˮ������Ϊ���Ĺؼ������ط���������������ƶ��������ۺϿ��飬��5��Ϊ�����ѵ㣬��Ŀ�ѶȽϴ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
�������ʱ���ᵼ���ռ����IJ�Ʒ�л��и߷е����ʵ�ͼʾ�ǣ�������
A�� |
B�� |
C�� |
D�� |
�����й���Һ���ʵ���������ȷ���ǣ�������
| A��ǿ�������ˮ���ܽ��һ������������� |
| B����һ������ͨ����������Һ������������������ |
| C��75mL 2mol/L�Ȼ����Һ��c��Cl-����50mL 1 mol/L�Ȼ�����Һ�е�c��Cl-����� |
| D��Ũ��Ϊ1mol/L�Ĵ�����ҺlL����ʹ��Ũ�ȱ�Ϊ2mol/L������Һ��������Ũ����0.5L |