��Ŀ����
�����йص������Һ�����ӵ����ʵ���Ũ�ȴ�С��ϵ��ȷ����( )
A.�����ʵ���Ũ�ȵ�������Һ����H2CO3����Na2CO3����NaHCO3����(NH4)2CO3�� ����c(CO32-)�Ĵ�С��ϵΪ����>��>��>��
B.pH=2 ��H2C2O4 ��Һ��pH=12 ��NaOH ��Һ�������ϣ� c(Na+)+c(H+) =c(OH-)+c(HC2O4-)+c(C2O42-)
C.��0.2 mol/LNaHCO3 ��Һ�м�������0.1 mol/LNaOH ��Һ�� c(CO32-)��c(HCO3-)��c(OH��)��c(H+)
D.�����£�ͬŨ�ȵ�CH3COONa ��CH3COOH ��Һ�������ϣ���Һ��pH��7�� c(CH3COOH)+c(OH-)��c(Na+)+c(H+)
��ϰ��ϵ�д�
�����Ŀ


 nZ(g)+2W(g)��5 minĩ������0.2 mol W������֪��ZŨ�ȱ仯����ʾ�ķ�Ӧ����Ϊ0.01 mol/( L��min)����������Ӧ��Z����ķ�Ӧ����ʽ�л�ѧ����ϵ��n��ֵ��
nZ(g)+2W(g)��5 minĩ������0.2 mol W������֪��ZŨ�ȱ仯����ʾ�ķ�Ӧ����Ϊ0.01 mol/( L��min)����������Ӧ��Z����ķ�Ӧ����ʽ�л�ѧ����ϵ��n��ֵ��
 C6+LiCoO2������˵������ȷ����
C6+LiCoO2������˵������ȷ����
 S2-+H3O+
S2-+H3O+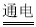 H2��+ Cl2��+2OH-
H2��+ Cl2��+2OH- ��SO
��SO ��Cl����CO
��Cl����CO �е�һ��(�����������в����ظ�����)����������ʵ�飺
�е�һ��(�����������в����ظ�����)����������ʵ�飺 ���ɣ�D����Һ������ɫ��ζ�������ݳ���
���ɣ�D����Һ������ɫ��ζ�������ݳ���