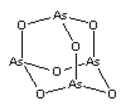
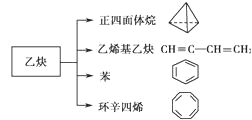
��Ŀ����
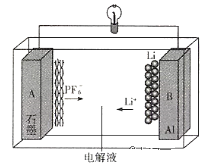
����Ŀ����ͼ��A����ȡ�屽��ʵ��װ�ã�B��C�ǸĽ����װ�ã�����ϸ�������Ա�����װ�ã��ش��������⣺
A. B.
B. 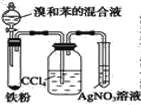 C.
C. 
(1)д������װ��������ͬ��������Ҫ��Ӧ�Ļ�ѧ����ʽ��__________________��������ķ�Ӧ�ķ�Ӧ����Ϊ________________
(2)װ�� A �� C �������˳��������ܣ���������______________��
(3)�ڰ�װ�� B װ��������ҩƷ��Ҫʹ��Ӧ��ʼ��Ӧ��װ�� B ���еIJ�����_____________������ʽΪ C8H10�����ڱ���ͬϵ��Ľṹ��_______________�֡�
(4)װ�� B��C �Ϻõؽ���� A �м�װҩƷ��ʹװ�ü�ʱ�ܷ��ì�ܣ������˲�����A װ������һ������ʵ������ɵĺ����______________��
(5)B �в�����ϴ��ƿ����װ�ã���������_______________����Ӧ��ϴ��ƿ�п��ܳ��ֵ�������_________________��
(6)B װ��Ҳ�����������Ե�ȱ�㣬ʹʵ���Ч�����û����������С�������ȱ����_________��
���𰸡�2Fe + 3Br2 = 2FcBr3��![]() ȡ����Ӧ ����HBr���������������� B��������Һ©���Ļ�����ʹ��ͱ��Ļ��Һ���������� 4 ��ͱ������ݳ�����Ⱦ���� ���շ�Ӧ����HBr�ݳ����������ͱ����� CCl4����ɫ��Ϊ��ɫ ����HBr�ݳ����������ͱ��������ܻ�������Ӧ���У�ԭ�������ʵͣ������ڵ��ܲ���AgNO3��Һ�ж��ײ�������
ȡ����Ӧ ����HBr���������������� B��������Һ©���Ļ�����ʹ��ͱ��Ļ��Һ���������� 4 ��ͱ������ݳ�����Ⱦ���� ���շ�Ӧ����HBr�ݳ����������ͱ����� CCl4����ɫ��Ϊ��ɫ ����HBr�ݳ����������ͱ��������ܻ�������Ӧ���У�ԭ�������ʵͣ������ڵ��ܲ���AgNO3��Һ�ж��ײ�������
��������
(1)A��B��C����װ�ö�����ȡ�屽,���ᷢ��������ķ�Ӧ���������ȡ����Ӧ��
(2)�������ӷ����������ܵ����������������ã�
(3)������ͱ��Ļ��Һ�����ۻ�ϲ��ܷ�����Ӧ������ʽΪ C8H10�����ڱ���ͬϵ��Ϊ�뱽���2��-CH2���ҽṹ���ƣ��ݴ��жϸ�����
(4) ����Br2�ͱ������ӷ�������Aװ�ò��ܼ�ʱ���ܷ⣬���Ի���ɱ����������ݳ�����Ⱦ�������ݴ˻ش�
(5) B��ϴ��ƿ��װ��CCl4�л��ܼ������������ձ���������������������CCl4���ʳ�ɫ������CCl4��Һ������ɫ��Ϊ��ɫ��
(6)��ԭ�������ʺ�ʵ�鰲ȫ�Ƕ�˼�������⡣
(1)����װ�þ����屽���Ʊ�װ�ã����Ʊ�ԭ��Ϊ��������Ӧ���廯��������Һ�����廯���Ĵ��������屽���廯�⣬��Ӧ�Ļ�ѧ����ʽΪ2Fe + 3Br2 = 2FcBr3��![]() �����б�������ķ�ӦΪȡ����Ӧ���ʴ�Ϊ��2Fe + 3Br2 = 2FcBr3��
�����б�������ķ�ӦΪȡ����Ӧ���ʴ�Ϊ��2Fe + 3Br2 = 2FcBr3��![]() ��ȡ����Ӧ��
��ȡ����Ӧ��
(2) A��C�г����ܵ������ǽ���Ӧ���ɵ�HBr���嵼��ˮ�У����ڸ÷�Ӧ���ȣ����Գ�����Ҳ�������������ã��ʴ�Ϊ������HBr���������������ã�
(3)װ��B���Կ��Ʒ�Ӧ�ķ�����ֹͣ��Bװ��ͨ����Һ©�����ƣ�������Һ©��������ʱ����Ӧ��ʼ������ʽΪ C8H10�����ڱ���ͬϵ��Ľṹ�У��Զ��ױ����ڶ��ױ�������ױ���������4�֣��ʴ�Ϊ��B��������Һ©���Ļ�����ʹ��ͱ��Ļ��Һ���������ϣ�4��
(4)����Br2�ͱ������ӷ�������Aװ�ò��ܼ�ʱ���ܷ⣬���Ի���ɱ����������ݳ�����Ⱦ�������ʴ�Ϊ����ͱ������ݳ�����Ⱦ������
(5)B��ϴ��ƿ��װ��CCl4�л��ܼ������������ձ���������������������CCl4���ʳ�ɫ������CCl4��Һ������ɫ��Ϊ��ɫ���ʴ�Ϊ�����շ�Ӧ����HBr�ݳ����������ͱ�������CCl4����ɫ��Ϊ��ɫ��
(6)Bװ���д��ڵ���Ҫ����������ڱ��������������Ļӷ��Ҳ��ܻ������Ӷ�����ԭ���ϵ��˷ѣ������ʽ��ͣ������ֱ�Ӳ���AgNO3��Һ�У�����ӦͻȻֹͣ��������ѹǿͻȻ���ͣ����������������ʴ�Ϊ������HBr�ݳ����������ͱ��������ܻ�������Ӧ���У�ԭ�������ʵͣ������ڵ��ܲ���AgNO3��Һ�ж��ײ���������