��Ŀ����
�����������ΰ�һ��������Ϻ��ۣ����Ƶû�����X��X����ˮ�ܵ����K+��Cr3+��SO42-������2.83 g X�е�Cr3+ȫ������ΪCr2O72-����Һ�е�Cr2O72-�ɺ���KI��Һ��Ӧ���õ�3.81 g I2����Ӧ�����ӷ���ʽΪ��Cr2O72-+6I-+14H+=2Cr3++3I2+7H2O����������2.83 g X����Һ�У����������BaCl2��Һ���ɵõ�4.66 g��ɫ�������ɴ˿��ƶϳ�X�Ļ�ѧʽΪ�� ��
A��K2SO4��2Cr2(SO4)3 B��2K2SO4��Cr2(SO4)3
C��K2SO4��Cr2(SO4)3 D��K2SO4��1/2Cr2(SO4)3
C
����:��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 ���ӣ��ֽ�2.83g W�е�Cr3+����ȫ��������
���ӣ��ֽ�2.83g W�е�Cr3+����ȫ��������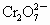 ���ӣ��ٺ���KI��Һ��Ӧ(
���ӣ��ٺ���KI��Һ��Ӧ( ��6I����14H+
��6I����14H+ 2Cr3+��3I2��7H2O)�õ�3.81g I2����ȡ2.83g WͶ�����BaCl2��Һ�У��õ�4.66g�������ɴ��ж�W�Ļ�ѧʽΪ��
2Cr3+��3I2��7H2O)�õ�3.81g I2����ȡ2.83g WͶ�����BaCl2��Һ�У��õ�4.66g�������ɴ��ж�W�Ļ�ѧʽΪ�� ���ֽ�2.83g W�е�Cr3+ȫ������Ϊ
���ֽ�2.83g W�е�Cr3+ȫ������Ϊ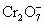 ����Щ
����Щ