题目内容
【题目】硒(Se)是第34号元素,是人体内不可或缺的微量元素,可以形成H2Se、SeO2、H2SeO3、H2SeO4、CuSe等多种化合物。请回答下列问题:
(1)硒在元素周期表中的位置________________________。
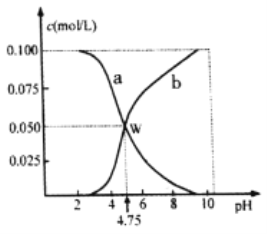
(2)在101kPa、一定温度(一般是298K)下,由稳定单质发生反应生成1mol化合物的反应热叫该化合物的标准生成热(△fHθ)。图1为氧族元素氢化物a、b、c、d呈气态时的生成热数据。
①图1中氢化物d的电子式为__________________________。
②在298K时,硒化氢分解反应的热化学反应方程式为__________________________。
③由图中数据计算,2H2Se(g)+O2(g) ![]() 2Se(s)+2H2O(g) △H=_____________KJ/mol
2Se(s)+2H2O(g) △H=_____________KJ/mol
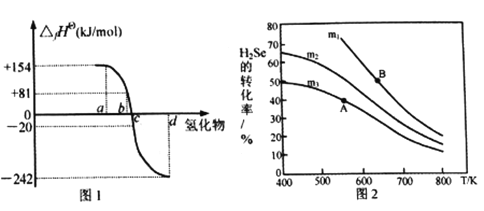
(3)在恒容反应器中,将H2Se(g)和O2(g)按不同比例[n(H2Se)/n(O2)=m]投入反应器,测得反应2H2Se(g)+O2(g) ![]() 2Se(s)+2H2O(g)中H2Se的平衡转化率随温度变化如图2所示。则A、B两点平衡常数大小关系为KA________KB(填“<”、“>”或“=”),图中m1、m2、m3由大到小的顺序为 ____________,理由是____________________________________。
2Se(s)+2H2O(g)中H2Se的平衡转化率随温度变化如图2所示。则A、B两点平衡常数大小关系为KA________KB(填“<”、“>”或“=”),图中m1、m2、m3由大到小的顺序为 ____________,理由是____________________________________。
(4)常温下溶度积:Ksp(CuSe)=7.9x10-49,Ksp(CuS)=1.3×10-36。则反应CuS(s)+Se2-(aq) ![]() CuSe(s)+S2-(aq)的化学平衡常数K为____________(结果用科学记数法表示,并保留2位小数)。当溶液中c(S2-)=100c(Se2-)时,反应中v(正)_____v(逆)(填“<”、“>”或“=”) 。
CuSe(s)+S2-(aq)的化学平衡常数K为____________(结果用科学记数法表示,并保留2位小数)。当溶液中c(S2-)=100c(Se2-)时,反应中v(正)_____v(逆)(填“<”、“>”或“=”) 。
【答案】 第四周期ⅥA族 ![]() H2Se(g)=H2(g)+Se(s)△H=-81kJ/mol -646 > m3>m2>m1 相同温度下,增大O2(g)的浓度,m值减小,平衡正向移动,H2Se的平衡转化率增大 1.65×1012 >
H2Se(g)=H2(g)+Se(s)△H=-81kJ/mol -646 > m3>m2>m1 相同温度下,增大O2(g)的浓度,m值减小,平衡正向移动,H2Se的平衡转化率增大 1.65×1012 >
【解析】(1)硒在元素周期表中的位置为:第四周期ⅥA族;
(2)非金属元素氢化物的稳定性与生成1mol氢化物时的△H的关系为:根据元素周期律,同一主族元素非金属性越强,生成气态氢化物越容易,气态氢化物越稳定;而根据热力学,能量越低越稳定.a、b、c、d依次为H2Te、H2Se、H2S、H2O。
①d的电子式为![]() 。
。
②由图1可知,在298K时,硒化氢的生成热是81kJ/mol,所以分解热△H=-81kJ/mol,故分解反应的热化学反应方程式为:H2Se(g)=H2(g)+Se(s)△H=-81kJ/mol
③由图1可知,H2O的生成热的热方程式为H2+1/2O2=H2O △H=-242kJ/mol(1),H2Se分解反应的热化学反应方程式为:H2Se(g)=H2(g)+Se(s)△H=-81kJ/mol(2),根据盖斯定律,(1)×2+(2)×2可得目标方程式,故△H=-242×2-81×2=-646 kJ/mol。
(3)由图2可知,升温转化率降低,则升温平衡常数减小,B点温度高于A点,故KA>KB;相同温度下,H2Se的平衡转化率m3<m2<m1,结合n(H2Se)/n(O2)=m,相同温度下,增大O2(g)的浓度,m值减小,平衡正向移动,H2Se的平衡转化率增大,所以m3>m2>m1。
(4)根据已知方程式可以得出化学平衡常数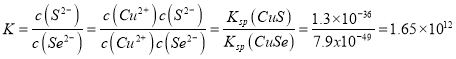 ;当哈学平衡时有
;当哈学平衡时有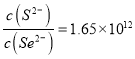 ,而此时溶液中
,而此时溶液中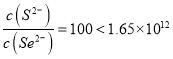 反应还没有平衡并且v(正)>v(逆),故答案为:1.65×1012; >。
反应还没有平衡并且v(正)>v(逆),故答案为:1.65×1012; >。