��Ŀ����
I.���ݼ۲���ӶԻ������ۣ�VSEPR�������жϷ��ӻ����ӵĿռ乹�͡�
��ش��������⣺
�ٽ���CO2Ϊֱ���ͷ��ӵ�ԭ�� ��
�� PbCl2���ӵ����幹��Ϊ ���÷������� ���ӣ�����ԡ��Ǽ��ԡ� ��������Pbԭ�ӵ��ӻ��������Ϊ ��
II.ͭ���ҹ���ɫ�������ϵ������н����������㷺��Ӧ���ڵ�������е���졢���������ش��������⣺
��1��ͭԭ�ӻ�̬�����Ų�ʽΪ ��
��2���þ����X�������䷨���Բ�ð���٤���������Խ���ͭ�IJⶨ�õ����½��������Ϊ�����������ܶѻ����߳�Ϊ361pm����֪ͭ���ܶ�Ϊ9.00g/cm3,��ͭ�����������
cm3�������������� �ˣ�����٤������Ϊ
����ʽ���㣬��֪Ar��Cu��=63.6����
��ش��������⣺
�ٽ���CO2Ϊֱ���ͷ��ӵ�ԭ�� ��
�� PbCl2���ӵ����幹��Ϊ ���÷������� ���ӣ�����ԡ��Ǽ��ԡ� ��������Pbԭ�ӵ��ӻ��������Ϊ ��
II.ͭ���ҹ���ɫ�������ϵ������н����������㷺��Ӧ���ڵ�������е���졢���������ش��������⣺
��1��ͭԭ�ӻ�̬�����Ų�ʽΪ ��
��2���þ����X�������䷨���Բ�ð���٤���������Խ���ͭ�IJⶨ�õ����½��������Ϊ�����������ܶѻ����߳�Ϊ361pm����֪ͭ���ܶ�Ϊ9.00g/cm3,��ͭ�����������
cm3�������������� �ˣ�����٤������Ϊ
����ʽ���㣬��֪Ar��Cu��=63.6����
I. (1) ��CO2��n��m��2��SP�ӻ�����Ϊֱ���� ��ÿ��2�֣�
�������Σ���V�Σ� ���Է��� SP2 ��ÿ��1�֣�
II.��1��1s22s22p63s23p63d104s1 ��2��4.70��10-23 4.23��10-22
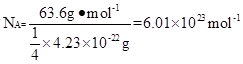
�������Σ���V�Σ� ���Է��� SP2 ��ÿ��1�֣�
II.��1��1s22s22p63s23p63d104s1 ��2��4.70��10-23 4.23��10-22
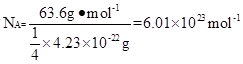
I. (1)CO2������ԭ��̼ԭ�ӵŶԵ��Ӷ����ǣ�4��2��2����2��0����̼ԭ����sp�ӻ���������ֱ���ͽṹ��
��PbCl2������ԭ��Pbԭ�ӵŶԵ��Ӷ����ǣ�4��1��2����2��1����Pbԭ����sp2�ӻ���������V�ͽṹ��
II.��1�����ݹ���ԭ����֪����̬ͭԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p63d104s1 ��
��2���߳���361pm�����������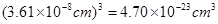 �������ܶȿ�֪������������
�������ܶȿ�֪������������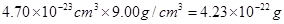 ������ͭ�����к��е�ͭԭ������8��1/8+6��1/2=4��������
������ͭ�����к��е�ͭԭ������8��1/8+6��1/2=4��������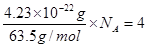 �����
�����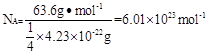 ��
��
��PbCl2������ԭ��Pbԭ�ӵŶԵ��Ӷ����ǣ�4��1��2����2��1����Pbԭ����sp2�ӻ���������V�ͽṹ��
II.��1�����ݹ���ԭ����֪����̬ͭԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p63d104s1 ��
��2���߳���361pm�����������
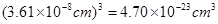 �������ܶȿ�֪������������
�������ܶȿ�֪������������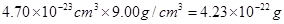 ������ͭ�����к��е�ͭԭ������8��1/8+6��1/2=4��������
������ͭ�����к��е�ͭԭ������8��1/8+6��1/2=4��������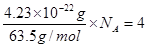 �����
�����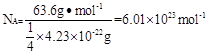 ��
��
��ϰ��ϵ�д�
 �Ƹ�С״Ԫ�������������ϵ�д�
�Ƹ�С״Ԫ�������������ϵ�д� ����һ������ܼƻ�ϵ�д�
����һ������ܼƻ�ϵ�д�
�����Ŀ
 ����CH��CH ��NH3����CH4
����CH��CH ��NH3����CH4