��Ŀ����
��15�֣�ʵ�����������᳧����(��Ҫ�ɷ�Ϊ���������P����FeS��SiO2��)�Ʊ�����(��ʽ�������ľۺ���)���̷�(FeSO4��7H2O)���������£�
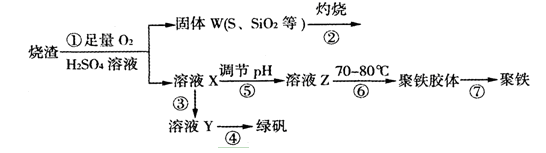
��1�������̢��еIJ���������ͨ��������Һ�У���Һ����ɫ���� ��
A��Ʒ����Һ B����ɫʯ����Һ C������KMnO4��Һ D����ˮ
��2�����̢��У�FeS��O2��H2SO4��Ӧ�����ӷ���ʽΪ�� ��
��3�����̢��У������������� ��
��4�����̢��У������ᾧ��Ҫʹ�þƾ��ơ����Ǽܡ������ǣ�����Ҫ�������� ��
��5�����̢ݵ���pH��ѡ�������Լ��е� (��ѡ�����)��
A��ϡ���� B��CaCO3 C��NaOH��Һ
��6�����̢��У�����ҺZ���ȵ�70һ80�棬Ŀ���� ��
��7��ʵ����Ϊ�������õ��ľ�����Ʒ����Ԫ�ص�������������������ʵ�顣���÷�����ƽ��ȡ2.700g��Ʒ���ڽ���Ʒ�����������������������Ȼ�����Һ���۹��ˡ�ϴ�ӡ�����������ù�������Ϊ3.495g�����þ�����Ҫ�ɷ�Ϊ[(Fe(OH)(SO4)]n����þ�����Ʒ����Ԫ�ص���������Ϊ ��(���������в�����Ԫ�غ���Ԫ��)��

��1�������̢��еIJ���������ͨ��������Һ�У���Һ����ɫ���� ��
A��Ʒ����Һ B����ɫʯ����Һ C������KMnO4��Һ D����ˮ
��2�����̢��У�FeS��O2��H2SO4��Ӧ�����ӷ���ʽΪ�� ��
��3�����̢��У������������� ��
��4�����̢��У������ᾧ��Ҫʹ�þƾ��ơ����Ǽܡ������ǣ�����Ҫ�������� ��
��5�����̢ݵ���pH��ѡ�������Լ��е� (��ѡ�����)��
A��ϡ���� B��CaCO3 C��NaOH��Һ
��6�����̢��У�����ҺZ���ȵ�70һ80�棬Ŀ���� ��
��7��ʵ����Ϊ�������õ��ľ�����Ʒ����Ԫ�ص�������������������ʵ�顣���÷�����ƽ��ȡ2.700g��Ʒ���ڽ���Ʒ�����������������������Ȼ�����Һ���۹��ˡ�ϴ�ӡ�����������ù�������Ϊ3.495g�����þ�����Ҫ�ɷ�Ϊ[(Fe(OH)(SO4)]n����þ�����Ʒ����Ԫ�ص���������Ϊ ��(���������в�����Ԫ�غ���Ԫ��)��
��1��ACD ��2��4FeS+3O2 + 12H+= 4Fe3++6H2O+4S ��3��Fe(����) ��4������������
��5��C��6���ٽ�Fe3+��ˮ�� ��7��31.1%
��5��C��6���ٽ�Fe3+��ˮ�� ��7��31.1%
�����������1������W�к���S��SiO2,�������ա����е�S���ΪSO2���壬SO2������Ư���ԣ���ʹƷ����Һ��ɫ��SO2�����л�ԭ�ԣ���ʹ����KMnO4��Һ����ˮ����������Ӧ��Ӧ����ɫ������ʹ��ɫʯ����Һ��Ϊ��ɫ�����ѡ��ΪA��C��D����2�����̢��У����ݿ�ͼ�еĸ������ʼ������غ㶨�ɺ͵����غ㡢����غ��֪��FeS��O2��H2SO4��Ӧ�����ӷ���ʽΪ4FeS+3O2 + 12H+= 4Fe3++6H2O+4S����3�����ڷ�����Ӧ�õ��������к���Fe3+.�������Ҫ��ȡ�̷�FeSO4�����Թ��̢��У�Ҫ���뻹ԭ��Fe�ۡ���4�����̢��У������ᾧ��Ҫʹ�þƾ��ơ����Ǽܡ������ǣ�����Ҫ����������������������5�����̢ݵ���pH��ʹ��Һ��pH����Ӧ�ü����ų�A����������CaCO3���������ᣬ�����������������Ca2+�����׳�ȥ����˿�ѡ�������Լ��е�NaOH��ѡ��ΪC����6�����̢��У�����ҺZ���ȵ�70һ80�棬Ŀ���Ǵٽ�Fe3+��ˮ��ʹѸ�ٲ�����������7��n(SO42-)=3.495g��233g/mol=0.015mol��n(Fe)= n(SO42-)= 0.015mol,����m(Fe)=0.015mol��56g/mol=0.84g�����Ըþ�����Ʒ����Ԫ�ص���������Ϊ��0.84g�� 2.700g����100%=31.1%��2�����ʡ����ӷ���ʽ����д����ѧʵ�����������Ԫ�ص����������ļ����֪ʶ��

��ϰ��ϵ�д�
�����Ŀ
