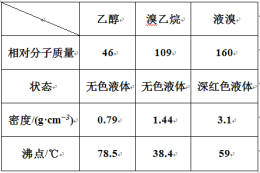
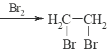��Ŀ����
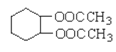
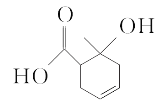
����Ŀ���ݿƼ��ձ��������Ͽ���ѧ�����Ŷӽ������ͱ������Ṳ�������״�ʵ�ֱ�ϩ����Ч����ɫ�ϳɡ���ϩ�����仯����ɺϳɸ��͡�ҽҩ��ũҩ�����ϣ��ϳ�ά����E��KI����Ȼ����ҩ����ɼ���ж����йؼ��ı�ϩ���ṹ����ϩ���Ľṹ��ʽΪCH2=CH-CH2OH����ش��������⣺
��1����̬��ԭ�ӵĵ����Ų�ʽΪ___��
��2��1molCH2=CH-CH2OH�������������ĸ�����Ϊ___����ϩ��������̼ԭ�ӵ��ӻ�����Ϊ___��
��3����ȩ(CH3CH2CHO�ķе�Ϊ49�棬��ϩ��(CH2=CHCH2OH)�ķе�Ϊ91�棬������Է���������ȣ��е����ϴ����Ҫԭ����___��
��4���ʻ���[Ni(CO)4]�����Ʊ��ߴ������ۣ������۵�Ϊ-25�棬�е�Ϊ43�档�ʻ�������������___��
��5��Ni2+���γɶ��������ӣ���[Ni(NH3)6]2+��[Ni(SCN)3]-��[Ni(CN)2]2-�ȡ�[Ni(NH3)6]2+����ԭ�ӵ���λ����___����SCN-��Ϊ�ȵ�����ķ���Ϊ___��
��6����NiO��������ͼ��ʾ��
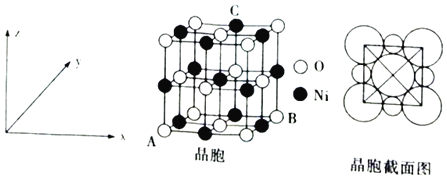
��������������ԭ�����������A(0��0��0)��B(1��1��0)����Cԭ���������Ϊ___��
����֪�������������ܶ�Ϊdgcm-3��NA���������ӵ�������ֵ����Ni2+�뾶Ϊ___nm(�ô���ʽ��ʾ)��
���𰸡�[Ar]3d84s2 9��1 sp3��sp2 ��ϩ�����Ӽ������� ���Ӿ��� 6 CO2�� (![]() ��1��1)
��1��1) ![]() ��3
��3![]() ��107
��107
��������
��1����̬��ԭ�Ӻ�����28�����ӣ����ݹ���ԭ����дNiԭ�Ӻ�������Ų�ʽ��
��2�����۵���Ϊ����������˫���к���1��������1����������1��CH2=CH-CH2OH�к���9��������1����������ϩ�������������ǻ���Cԭ�Ӽ۲���ӶԸ�����4������̼̼˫�����˵�̼ԭ�Ӽ۲���ӶԸ�����3�����ݼ۲���ӶԻ��������ж�̼ԭ�ӵ��ӻ����ͣ�
��3�����з��Ӽ�����������۷е�ϸߣ�
��4�����Ӿ����۷е�ϵͣ�
��5��[Ni��NH3��6]2+����ԭ�ӵ���λ����6����SCN-��Ϊ�ȵ�����ķ����к���3��ԭ�ӡ��۵�������16��
��6����������������ԭ�����������A��0��0��0����B��1��1��0����Cԭ����x��y��z��ֱ�Ϊ![]() ��1��1��
��1��1��
�����þ�̯����������������Ӹ����������Ӹ������ٸ���![]() �������������ݽ���ͼ�ҵ����Ӱ뾶�;��������Ĺ�ϵ������⡣
�������������ݽ���ͼ�ҵ����Ӱ뾶�;��������Ĺ�ϵ������⡣
![]() ��̬��ԭ�Ӻ�����28�����ӣ����ݹ���ԭ����дNiԭ�Ӻ�������Ų�ʽ
��̬��ԭ�Ӻ�����28�����ӣ����ݹ���ԭ����дNiԭ�Ӻ�������Ų�ʽ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
![]() ���۵���Ϊ
���۵���Ϊ![]() ��������˫���к���1��
��������˫���к���1��![]() ����1��
����1��![]() ������1��
������1��![]() ���9��
���9��![]() ����1��
����1��![]() ����1mol
����1mol![]() ��
��![]() ����
����![]() ���ĸ�����Ϊ9mol��
���ĸ�����Ϊ9mol��![]() ��1����ϩ�������������ǻ���Cԭ�Ӽ۲���ӶԸ�����4������̼̼˫�����˵�̼ԭ�Ӽ۲���ӶԸ�����3�����ݼ۲���ӶԻ��������ж�̼ԭ�ӵ��ӻ����ͣ�ǰ��Ϊ
��1����ϩ�������������ǻ���Cԭ�Ӽ۲���ӶԸ�����4������̼̼˫�����˵�̼ԭ�Ӽ۲���ӶԸ�����3�����ݼ۲���ӶԻ��������ж�̼ԭ�ӵ��ӻ����ͣ�ǰ��Ϊ![]() ������Ϊ
������Ϊ![]() ���ʴ�Ϊ��9��1��
���ʴ�Ϊ��9��1��![]() ��
��![]() ��
��
![]() ���з��Ӽ�����������۷е�ϸߣ���ϩ�����Ӽ��������������۷е�ϸߣ��ʴ�Ϊ����ϩ�����Ӽ���������
���з��Ӽ�����������۷е�ϸߣ���ϩ�����Ӽ��������������۷е�ϸߣ��ʴ�Ϊ����ϩ�����Ӽ���������
![]() ���Ӿ����۷е�ϵͣ��þ����۷е�ϵͣ�Ϊ���Ӿ��壬�ʴ�Ϊ�����Ӿ��壻
���Ӿ����۷е�ϵͣ��þ����۷е�ϵͣ�Ϊ���Ӿ��壬�ʴ�Ϊ�����Ӿ��壻
![]() ����ԭ�ӵ���λ����6����
����ԭ�ӵ���λ����6����![]() ��Ϊ�ȵ�����ķ����к���3��ԭ�ӡ��۵�������16����ȵ����������
��Ϊ�ȵ�����ķ����к���3��ԭ�ӡ��۵�������16����ȵ����������![]() �ȣ��ʴ�Ϊ��6��
�ȣ��ʴ�Ϊ��6��![]() �ȣ�
�ȣ�
![]() ������������ԭ�����������
������������ԭ�����������![]() 0��
0��![]() ��
��![]() 1��
1��![]() ��Cԭ����x��y��z��ֱ�Ϊ
��Cԭ����x��y��z��ֱ�Ϊ![]() ��1��1����C�㾧������Ϊ
��1��1����C�㾧������Ϊ![]() ��1��
��1��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��1��
��1��![]() ��
��
![]() �þ����У������Ӹ���
�þ����У������Ӹ���![]() �������Ӹ���
�������Ӹ���![]() ��
��
�����������ܶ�Ϊ![]() ��
��![]() ���������ӵ�������ֵ���������
���������ӵ�������ֵ��������� �������ⳤ
�������ⳤ![]() ������Խ��߳���
������Խ��߳���![]() ��
��![]() �뾶Ϊ
�뾶Ϊ![]() �������Ӱ뾶
�������Ӱ뾶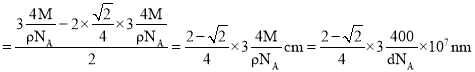 ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��

����Ŀ��X��Y��������������Z��Һ�й�����ͼ��װ�ã�ʵ���е�����ָ�뷢��ƫת��ͬʱX����֣�Y����ϸ����X��Y��Z������( )

ѡ�� | X | Y | Z |
A | Zn | Cu | ϡ���� |
B | Cu | Zn | ϡ���� |
C | Cu | Ag | ����ͭ��Һ |
D | Ag | Zn | ��������Һ |
A.AB. BC. CD. D