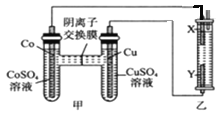
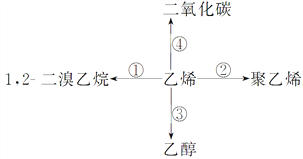
ЬтФПФкШн
ЁОЬтФПЁПI гавЛжжгаЛњЮяXЃЌЦфНсЙЙМђЪНЮЊЃКHOЁЊCH2CH=CHЁЊCOOHЃЌЪдЛиД№ЯТСаЮЪЬтЃК
(1)XжаЕФКЌбѕЙйФмЭХУћГЦЪЧ_______________ЁЂ_______________ЁЃ
(2)ЯђXжаМгШыН№ЪєФЦЃЌНЋЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЪЧ_______________ЁЃ
(3)ШчЙћдкXжаМгШыNaOHШмвКЃЌНЋЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЪЧ___________ЁЃ
(4)ЯТСаЙигкXЕФЫЕЗЈжае§ШЗЕФЪЧ________________ЁЃ
ЂйXМШФмгыЫсЗЂЩњѕЅЛЏЗДгІЃЌгжФмгыДМЗЂЩњѕЅЛЏЗДгІ
ЂкXФмЙЛЪЙфхЫЎЭЪЩЋЃЌЕЋВЛФмЪЙKMnO4ЫсадШмвКЭЪЩЋ
ЂлXФмЙЛЗЂЩњЫѕОлЗДгІЃЌЕЋВЛФмЗЂЩњМгОлЗДгІ
II.ЗжзгЪНЮЊC3H6O2ЕФгаЛњЮягаЖржжЭЌЗжвьЙЙЬхЃЌЯжгаЦфжаЕФЫФжжXЁЂYЁЂZЁЂWЃЌЫќУЧЕФЗжзгжаОљКЌМзЛљЃЌНЋЫќУЧЗжБ№НјааЯТСаЪЕбщвдМјБ№ЃЌЦфЪЕбщМЧТМШчЯТЃК
NaOHШмвК | вјАБШмвК | аТжЦCu(OH)2 | Н№ЪєФЦ | |
X | жаКЭЗДгІ | ЮоЯжЯѓ | ШмНт | ВњЩњЧтЦј |
Y | ЮоЯжЯѓ | гавјОЕ | МгШШКѓгазЉКьЩЋГСЕэ | ВњЩњЧтЦј |
Z | ЫЎНтЗДгІ | гавјОЕ | МгШШКѓгазЉКьЩЋГСЕэ | ЮоЯжЯѓ |
W | ЫЎНтЗДгІ | ЮоЯжЯѓ | ЮоЯжЯѓ | ЮоЯжЯѓ |
ЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉаДГіXЕФНсЙЙМђЪН________ЃЌ WЕФЯЕЭГУќУћЪЧ________.
ЃЈ2ЃЉЂйYдквЛЖЈЬѕМўЯТЗЂЩњЗжзгФкЕФЭбЫЎЗДгІЕФЛЏбЇЗНГЬЪН________________
ЂкZгыNaOHШмвКЗДгІЕФЛЏбЇЗНГЬЪН_______________________________
ЁОД№АИЁП єЧЛљ єШЛљ HOЁЊCH2CH=CHЁЊCOOH+2NaЁњNaOЁЊCH2CH=CHЁЊCOONa+H2Ёќ HOЁЊCH2CH=CHЁЊCOOH+NaOHЁњHOЁЊCH2CH=CHЁЊCOONa+H2O Ђй CH3CH2COOH ввЫсМзѕЅ ![]()
![]() CH2=CHCHOЃЋH2O HCOOCH2CH3ЃЋNaOH
CH2=CHCHOЃЋH2O HCOOCH2CH3ЃЋNaOH![]() HCOONaЃЋCH3CH2OH
HCOONaЃЋCH3CH2OH
ЁОНтЮіЁП(1)HO-CH2CHЈTCH-COOHжаКЌбѕЙйФмЭХЮЊєЧЛљКЭєШЛљЃЌЙЪД№АИЮЊЃКєЧЛљЁЂєШЛљЃЛ
(2)єЧЛљЁЂєШЛљЖМПЩгыФЦЗДгІЩњГЩЧтЦјЃЌЗНГЬЪНЮЊHO-CH2CHЈTCH-COOH+2NaЁњNaO-CH2CHЈTCH-COONa+H2ЁќЃЌЙЪД№АИЮЊЃКHO-CH2CHЈTCH-COOH+2NaЁњNaO-CH2CHЈTCH-COONa+H2ЁќЃЛ
(3)НсЙЙжажЛгаєШЛљгыЧтбѕЛЏФЦЗДгІЃЌЗНГЬЪНЮЊHO-CH2CHЈTCH-COOH+NaOH=HO-CH2CHЈTCH-COONa+H2OЃЌЙЪД№АИЮЊЃКHO-CH2CHЈTCH-COOH+NaOH=HO-CH2CHЈTCH-COONa+H2OЃЛ
(4)ЂйвђЮЊXКЌгаєЧЛљКЭєШЛљЃЌдђМШФмгыЫсЗЂЩњѕЅЛЏЗДгІЃЌгжФмгыДМЗЂЩњѕЅЛЏЗДгІЃЌЙЪе§ШЗЃЛЂкXКЌгаЬМЬМЫЋМќЃЌПЩЗЂЩњМгГЩЗДгІКЭбѕЛЏЗДгІЃЌЙЪДэЮѓЃЛЂлXКЌгаЬМЬМЫЋМќЃЌПЩЗЂЩњМгОлЗДгІЃЌЙЪДэЮѓЃЛЙЪД№АИЮЊЃКЂйЁЃ
II.ЗжзгЪНЮЊC3H6O2ЕФгаЛњЮяXЁЂYЁЂZЁЂW,ЫќУЧЕФЗжзгжаОљКЌМзЛљ,гЩXФмгыNaOHЗЂЩњжаКЭЗДгІЧвФмШмНтCu(OH)2,ЫЕУїКЌга-COOH,дђXЮЊCH3CH2COOHЃЛYФмЗЂЩњвјОЕЗДгІ,ЫЕУїКЌга-CHO,ФмгыNaЗЂЩњЗДгІЩњГЩЧтЦј,ЫЕУїКЌга-OH,YЮЊCH3CHOHCHO;ZМШФмЗЂЩњЫЎНт,гжФмЗЂЩњвјОЕЗДгІ,ЫЕУїКЌга-OOCH,ЮЊМзЫсѕЅ,МДZЮЊHCOOCH2CH3,WФмЫЎНт,КЌгаѕЅЛљ,WЮЊCH3COOCH3,(1)гЩЩЯЪіЗжЮіПЩвджЊЕР,XЮЊCH3CH2COOHЁЂЮЊWЁЂCH3COOCH3ЃЌ WЕФЮЊввЫсМзѕЅЃЌвђДЫЃЌБОЬте§ШЗД№АИЪЧ: CH3CH2COOH;ввЫсМзѕЅЁЃ
(2)ЂйYЮЊCH3CHOHCHO ЃЌдђYдквЛЖЈЬѕМўЯТЗЂЩњЗжзгФкЕФЭбЫЎЗДгІЕФЛЏбЇЗНГЬЪНЃК. ![]()
![]() CH2=CHCHOЃЋH2O
CH2=CHCHOЃЋH2O
ЂквђЮЊZЮЊHCOOCH2CH3ЃЌдђZгыNaOHШмвКЗДгІЕФЛЏбЇЗНГЬЪНЮЊ![]() ,вђДЫЃЌБОЬте§ШЗД№АИЪЧ:
,вђДЫЃЌБОЬте§ШЗД№АИЪЧ:![]()

 гЅХЩНЬИЈЯЮНгНЬВФКгББНЬг§ГіАцЩчЯЕСаД№АИ
гЅХЩНЬИЈЯЮНгНЬВФКгББНЬг§ГіАцЩчЯЕСаД№АИ