��Ŀ����
KClO3��ŨHCl��һ���¶��·�Ӧ�����ɻ���ɫ���ױ���������ȡ��䷴Ӧ�ɱ���Ϊ��
KClO3+ HCl(Ũ)�� KCl+ ClO2+ Cl2+ H2O
��1����ƽ���ϻ�ѧ����ʽ(��ѧ���������뻮����)����˫���ű������ת�Ƶķ������Ŀ��
��2����Ӧ�е��������� �� ClO2�Ƿ�Ӧ�� (��������ԭ)���
��3����Ӧ����0.1 mol Cl2����ת�Ƶĵ��ӵ����ʵ���Ϊ mol��
��4��ClO2���к�ǿ�������ԣ���˿ɱ�����������������ClO2����������������ת��ΪCl-������������Ч��(�Ե�λ���ʵ����õ���������ʾ)��Cl2�� ����
��1��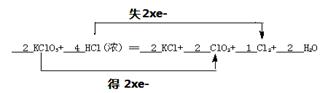 ��2�֣�
��2�֣�
��2��KClO3 ��1�֣� ��ԭ ��1�֣� ��3��0.2mol ��1�֣� ��4��2.5�� (1��)
���������������1��KClO3����Ԫ�ػ��ϼ���+5�۽���ΪClO2��+4�ۣ��õ�1�����ӣ����������������HCl����Ԫ�ػ��ϼ���-1������ΪCl2��0�ۣ�ʧȥ1�����ӣ��Ȼ����ǻ�ԭ�������ݵ��ӵ�ʧ�غ��֪���������뻹ԭ�������ʵ���֮����1:1�����KClO3ϵ��Ϊ2��ClO2ϵ��Ϊ2��KClϵ��Ϊ2��Cl2ϵ��Ϊ1����HClϵ��Ϊ4�����ԭ���غ��֪ˮ��ϵ����2����˸÷�Ӧ����ת�Ƶķ������Ŀ�ɱ�ʾΪ ��
��
��2����Ӧ2KClO3+4HCl��Ũ����2KCl+Cl2��+2ClO2��+2H2O�У���KClO3����Ԫ�ػ��ϼ��ɣ�1�۽���ΪClO2�У�4�ۿ�֪��KClO3����Ԫ�ر���ԭ������KClO3����������ClO2�ǻ�ԭ���
��3����Ӧ��ֻ��Cl2���������HCl����Ԫ�ػ��ϼ���-1������ΪCl2��0�ۣ����Բ���0.1molCl2ת�Ƶĵ��ӵ����ʵ���Ϊ0.1mol��2��0.2mol��
��4��1molCl2���Ի��2mol���ӣ�ClO2����������������ת��ΪCl-����˵��1molClO2���Ի��5mol���ӣ�����ClO2������Ч����Cl2�� ��2.5����
��2.5����
���㣺����������ԭ��Ӧ����ʽ����ƽ������������ԭ������ж��Լ��йؼ���

 ����������ϵ�д�
����������ϵ�д� �Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�2KMnO4+16HCl=2MnCl2+5Cl2��+8H2O+2KCl���������Ӧ�У��������� ������������ ����˫���ű�����ӵ�ת�Ʒ������Ŀ ��
�������ȣ�ClO2��Ϊһ�ֻ���ɫ���壬�ǹ����Ϲ��ϵĸ�Ч�����ס����١���ȫ��ɱ����������
��1����ҵ���Ʊ�ClO2�ķ�Ӧԭ�������ã�2NaClO3��4HCl=2ClO2����Cl2����2H2O��2NaCl��
��Ũ�����ڷ�Ӧ����ʾ������������_______������ţ���
| A��ֻ�л�ԭ�� | B����ԭ�Ժ����� | C��ֻ�������� | D�������Ժ����� |
��2��Ŀǰ�ѿ������õ�ⷨ��ȡClO2���¹��ա�
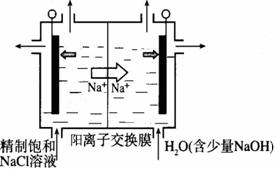
����ͼʾ����ʯī���缫����һ�������µ�ⱥ��ʳ��ˮ��ȡClO2��д����������ClO2�ĵ缫��Ӧʽ�� ��
�ڵ��һ��ʱ�䣬�������������������Ϊ112 mL����״����ʱ��ֹͣ��⡣ͨ�������ӽ���Ĥ�������ӵ����ʵ���Ϊ_________mol����ƽ���ƶ�ԭ������������pH�����ԭ�� ��
��3��ClO2����ˮ��Fe2����Mn2����S2����CN���������Ե�ȥ��Ч����ij������ˮ�к�
CN�� a mg/L������ClO2��CN-������ֻ�����������壬�����ӷ�Ӧ����ʽΪ ��
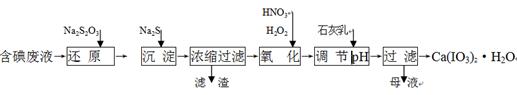
 HSO3- + H+�ĵ��볣��Ka= 1��10-2 mol/L������¶���NaHSO3��ˮ��ƽ�ⳣ��Kh= mol/L������NaHSO3��Һ�м���������I2������Һ��
HSO3- + H+�ĵ��볣��Ka= 1��10-2 mol/L������¶���NaHSO3��ˮ��ƽ�ⳣ��Kh= mol/L������NaHSO3��Һ�м���������I2������Һ��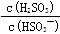 �� ���������С�����䡱����ͬ���� ��������NaOH��Һ��
�� ���������С�����䡱����ͬ���� ��������NaOH��Һ��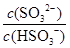 ��ֵ ����������ˮ��ˮ�ĵ���̶Ƚ� ��
��ֵ ����������ˮ��ˮ�ĵ���̶Ƚ� ��