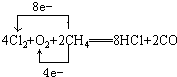��Ŀ����
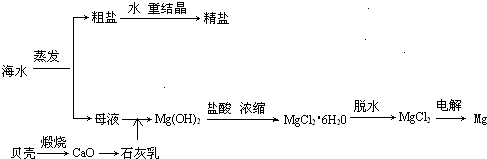
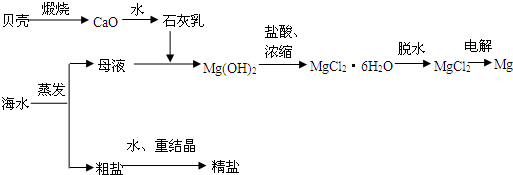
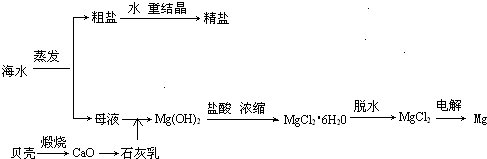
��ˮ���ۺ����ÿ����Ʊ�����þ������������ͼ��ʾ
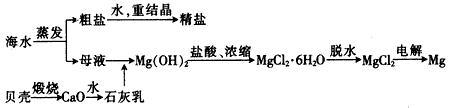
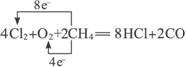
(1)���MgCl2ʱ������Mg�ĵ缫��_________�������������������
(2)ʵ�����ォ�����Ƴɾ��εĹ����У����ܽ⡢���ˡ�������������IJ����ж�Ҫ�õ������������й����в�������������_____________��
(3)���ڿ����м���MgCl2��6H2O���ɵ���Mg(OH)Cl��MgO��д����Ӧ��Ӧ�Ļ�ѧ����ʽ��_________________________����Ҫ�Ƶ���ˮMgCl2��������________�м���MgCl2��6H2O��
(4)Mg(OH)2�����л��е�Ca(OH)2Ӧ������ȥ��д���漰�ķ�Ӧ�Ļ�ѧ����ʽ��__________________��
(2)ʵ�����ォ�����Ƴɾ��εĹ����У����ܽ⡢���ˡ�������������IJ����ж�Ҫ�õ������������й����в�������������_____________��
(3)���ڿ����м���MgCl2��6H2O���ɵ���Mg(OH)Cl��MgO��д����Ӧ��Ӧ�Ļ�ѧ����ʽ��_________________________����Ҫ�Ƶ���ˮMgCl2��������________�м���MgCl2��6H2O��
(4)Mg(OH)2�����л��е�Ca(OH)2Ӧ������ȥ��д���漰�ķ�Ӧ�Ļ�ѧ����ʽ��__________________��
(1)��
(2)����
(3)MgCl2��6H2O Mg(OH)Cl+HCl��+5H2O����MgCl��6H2O
Mg(OH)Cl+HCl��+5H2O����MgCl��6H2O MgO+2HCl��+5H2O����HCl����
MgO+2HCl��+5H2O����HCl����
(4)Ca(OH)2+MgCl2=CaCl2+Mg(OH)2��
(2)����
(3)MgCl2��6H2O
 Mg(OH)Cl+HCl��+5H2O����MgCl��6H2O
Mg(OH)Cl+HCl��+5H2O����MgCl��6H2O MgO+2HCl��+5H2O����HCl����
MgO+2HCl��+5H2O����HCl���� (4)Ca(OH)2+MgCl2=CaCl2+Mg(OH)2��

��ϰ��ϵ�д�
 ϰ�⾫ѡϵ�д�
ϰ�⾫ѡϵ�д�
�����Ŀ