��Ŀ����
������Ȼ�糣��������ˮ����������Ca3(PO4)2����ʽ���ڡ����ĵ��ʺͻ��������Ź㷺��Ӧ�á�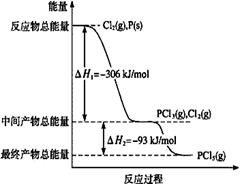
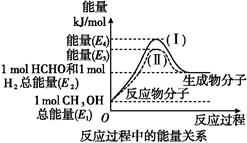
��1������P(s)��Cl2(g)������Ӧ����PCl3(g)��PCl5(g)����Ӧ���̺�������ϵ��ͼ��ʾ��ͼ�еġ�H��ʾ����1mol��������ݣ���
��ش����⣺
��PCl5�ֽ��PCl3��Cl2���Ȼ�ѧ����ʽ�� ��
��P��Cl2��������Ӧ����1 mol PCl5�ġ�H3�� ��
��2��PCl5�ֽ��PCl3��Cl2�ķ�Ӧ�ǿ��淴Ӧ��T��ʱ����2.0 L�����ܱ������г���1.0 mol PCl5������250 s�ﵽƽ�⡣��Ӧ�����вⶨ�IJ������ݼ��±���
| t / s | 0 | 50 | 150 | 250 | 350 |
| n(PCl3) / mol | 0 | 0��16 | 0��19 | 0��20 | 0��20 |
�ٷ�Ӧ��50��150s �ڵ�ƽ������v(PCl3)�� ��
���Լ�����¶��·�Ӧ��ƽ�ⳣ����д��������̣�����2λ��Ч���֣�
��3��NaH2PO4��Na2HPO4��Na3PO4��ͨ��H3PO4��NaOH��Һ��Ӧ��ã��������ֵķֲ�����(ƽ��ʱij���ֵ�Ũ��ռ������Ũ��֮�͵ķ���)��pH �Ĺ�ϵ��ͼ��ʾ��
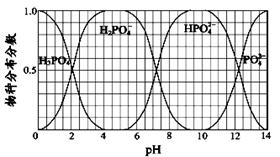
��Ϊ��ýϴ���Na2HPO4��pHӦ������ ��pH��6ʱ����Һ����Ҫ��������Ũ�ȴ�С��ϵΪ�� ��
��Na2HPO4��Һ�ʼ��ԣ�����������CaCl2��Һ����Һ�����ԣ���Һ�����Ե�ԭ���ǣ�������ƽ��Ƕȷ����� ��
��1����PCl5(g)=PCl3(g)+Cl2(g)��H����93kJ/mol��2�֣�����ʽ1�֣���H�ı�ʾ1�֣���ѧʽ��״̬����0�֣�+���ʱ���ֵ����λ��©�Ͽ�1�֣��������÷�����ʾ���ʱ���ƥ��Ҳ���֣�
�ڣ�399 kJ/mol��2�֣���λ��©��1�֣�
��2����1.5��10-4mol/(L��s) ��0.00015 mol/(L��s)��2�֣���λ��©��1�֣�
��2.5��10-2 mol/L��0.025mol/L
��3����9��10.5��2�֣����ڴ�����������ڵ�ijһ�㣩
c(H2PO4��)��c(HPO42��) ��2�֣�
��Na2HPO4��Һ�д��ڵ���ƽ�⣬HPO42�� H++PO43����1�֣�������CaCl2��Һ��Ca2����PO43���������Ca3(PO4)2��������ʹNa2HPO4����ƽ�������ƶ���H+Ũ��������Һ�����ԣ�1�֣��������������֣�
H++PO43����1�֣�������CaCl2��Һ��Ca2����PO43���������Ca3(PO4)2��������ʹNa2HPO4����ƽ�������ƶ���H+Ũ��������Һ�����ԣ�1�֣��������������֣�
���������������1����������ر�ע��ͼ������ת�����ʱ�������1mol��������ݣ����PCl5�ֽ��PCl3��Cl2�����ȷ�Ӧ�����Ȼ�ѧ����ʽΪ��PCl5(g)=PCl3(g)+Cl2(g)��H����93kJ/mol��
�ڼ���P��Cl2��������Ӧ����1 mol PCl5 ���ʱ䣬���ݸ�˹���ɷ�Ӧ���ʱ�������أ�ֻ�轫ͼ���������ֵ��ʱ���Ӽ��ɣ����ԡ�H3����399 kJ/mol��
��2���ټ��㷴Ӧ��50��150s �ڵ���PCl3��ʾ�ķ�Ӧ���ʣ�ֻ������ݴ��빫ʽ�������v(PCl3)����C/��t=(0��19-0��16)mol/(2L��100s)= 1.5��10-4mol/(L��s)��
�� PCl5(g) = PCl3(g)+Cl2(g)
��ʼŨ�ȣ�mol/L�� 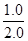 =0.50 0 0
=0.50 0 0
ת��Ũ�ȣ�mol/L�� 0.10 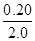 =0.10 0.10
=0.10 0.10
ƽ��Ũ�ȣ�mol/L�� 0.40 0.10 0.10 ��1�֣�
K�� 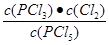 =
=  �� 2.5��10-2 mol/L��0.025mol/L
�� 2.5��10-2 mol/L��0.025mol/L
��3������Ҫѧ�������ͼ����ͼ��ʾ����H3PO4�벻�ϼ����NaOH��Ӧ��������������������ʹ��ҺpHֵ��������ʱ����Һ�и��������ֵİٷֱȡ�ÿ������������һ����PH��Χ�ڶ��аٷֺ�������ͼ�С��������������߽��沿�ֱ�ʾ������2�ֺ�������ͬʱ���ڵ������
��Ҫ��ýд�����Na2HPO4����ͨ��ͼ���ҵ�PHֵ��9~10.5�ķ�Χ�ڣ�HPO42- �İٷֺ����ӽ��ٷְ٣����Կ��Ƶ�PHֻҪ�������Χ�ڣ������Ի�ýϴ�����Na2HPO4 ��
��Na2HPO4��������ʽ�Σ�HPO42-���ڵ����ˮ������ƽ�⣬������Һ�ʼ��ԣ����Կ���ˮ��ǿ�ڵ��롣���Ǹ�����Һ�����Ȼ��ƺ���Һ�����ԣ���˿����Ʋ������Ȼ���һ���ı��˵���ƽ�⣬ʹ֮���ϲ����������ӣ�ʹ��Һ�����ԡ����Խ�ϱ������ɡ�������Ȼ�糣��������ˮ����������Ca3(PO4)2����ʽ���ڡ������Խ���ƽ����ƶ�Ϊ��Na2HPO4��Һ�д��ڵ���ƽ�⣬HPO42�� H++PO43��������CaCl2��Һ��Ca2����PO43���������Ca3(PO4)2��������ʹNa2HPO4����ƽ�������ƶ���H+Ũ��������Һ�����ԡ�
H++PO43��������CaCl2��Һ��Ca2����PO43���������Ca3(PO4)2��������ʹNa2HPO4����ƽ�������ƶ���H+Ũ��������Һ�����ԡ�
���㣺���⿼����Ƿ�Ӧԭ����֪ʶ�������ڻ�ѧ�뷴Ӧ�����Ĺ�ϵ����Ӧ���ʺ�ƽ�ⳣ�����㡢ƽ���ƶ�ԭ�����͵ȷ���Ŀ��顣

 ���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д��״���һ�ֿ�����������ȼ�ϣ���;�㷺���о������þ��й���ǰ����
��1����֪�ڳ��³�ѹ�£���÷�Ӧ�ķ�Ӧ�����£�
�� 2CH3OH(l)+ 3O2(g)  2CO2(g) +4H2O(g) ?H1= ��1275��6 kJ/mol
2CO2(g) +4H2O(g) ?H1= ��1275��6 kJ/mol
�� 2CO(g) +O2(g)  2CO2(g) ?H2=��566��0 kJ/mol
2CO2(g) ?H2=��566��0 kJ/mol
CH3OH����ȫȼ������CO����̬ˮ���Ȼ�ѧ����ʽ�� ��
��2����ҵ�������״��ķ�Ӧ���£�CO2(g) + 3H2(g)  CH3OH(g)+ H2O(g) ?H = ��49 kJ/mol
CH3OH(g)+ H2O(g) ?H = ��49 kJ/mol
��ij�¶��£��ݻ���Ϊ1 L��A��B���������У�����ͬ��ʽͶ�뷴Ӧ����ֺ��º��ݡ�����B�о�10 s��ﵽƽ�⡣�ﵽƽ��ʱ���й��������±���
| ���� | A | B |
| ��Ӧ��Ͷ���� | 1 mol CO2(g)��3 mol H2(g) | 1 mol CH3OH(g)��1 mol H2O(g) |
| ��Ӧ�����仯 | �ų���kJ���� | ����19��6 kJ���� |
�ٴӷ�Ӧ��ʼ���ﵽƽ��ʱ������B��CH3OH��ƽ����Ӧ����Ϊ ��
�ڸ��¶��£�B�����з�Ӧ�Ļ�ѧƽ�ⳣ������ֵΪ ��
�ۦ��� ��
�����д�ʩ��ʹ����A�м״��IJ���������� ��
a�������¶� b����ˮ��������ϵ����
c���ø���Ч�Ĵ��� d�����������ݻ���Сһ��
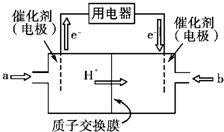
��3���ҹ���ѧԺ��ѧ�о����ڼ״�ȼ�ϵ�ؼ�����������ͻ�ƣ���װ�����Ժ�����ء��״�ȼ�ϵ�صĹ���ԭ������ͼ��ʾ��
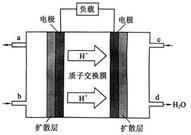
�� �õ�ع���ʱ��b��ͨ�������Ϊ ��
�� �õ�������ĵ缫��ӦʽΪ ��
��ú��Ϊȼ�Ͽ�ͨ����������;����
;��I�� C(s)��O2(g) = CO2(g)
;��II������ˮú���� C(s) + H2O(g) =" CO(g)" + H2(g)
ȼ��ˮú����2 CO(g) + O2(g) = 2CO2(g)��
2H2(g)+O2(g) =2H2O(g)
��֪����C(s)��O2(g)=CO2(g)����H1=��393.5 kJ��mol-1
��H2(g)+1/2O2(g)=H2O(g)����H2=��241.8kJ��mol-1
��CO(g)+ 1/2O2 (g) =CO2(g)����H3=��283.0kJ��mol-1
��ش��������⣺
��1��CO(g) + H2O(g) = H2(g) + CO2(g) �� ������ȷ�Ӧ�������ȷ�Ӧ����
��2�����ݸ�˹���ɣ�ú����̬ˮ����ˮú���ķ�Ӧ�ȡ�H= ��
��3����������;��������˵��������ǣ� ��
| A��;��II��ˮú��ʱ�����ܺģ���;��II����������ȡ |
| B����;��I��ȣ�;��II���Լ��ٶԻ�������Ⱦ |
| C����;��I��ȣ�;��II�������ú��ȼ��Ч�� |
| D����úת��Ϊˮú������ͨ���ܵ��������� |
 H+(aq) +CH3COO-(aq) ��H2="+1.3" kJ/mol
H+(aq) +CH3COO-(aq) ��H2="+1.3" kJ/mol CO2(g)����H1<0��
CO2(g)����H1<0�� CO2(g) + H2(g)����Ӧ�����и����ʵ�Ũ������ͼt1ǰ��ʾ�仯���������¶Ȳ��䣬t2ʱ���������г���CO��H2��1mol��ƽ�⽫ �ƶ�������� �����ҡ���������t2ʱ�����ı䷴Ӧ����������H2Ũ�ȷ�������ͼt2����ʾ�ı仯����ı������������ ������ţ���
CO2(g) + H2(g)����Ӧ�����и����ʵ�Ũ������ͼt1ǰ��ʾ�仯���������¶Ȳ��䣬t2ʱ���������г���CO��H2��1mol��ƽ�⽫ �ƶ�������� �����ҡ���������t2ʱ�����ı䷴Ӧ����������H2Ũ�ȷ�������ͼt2����ʾ�ı仯����ı������������ ������ţ���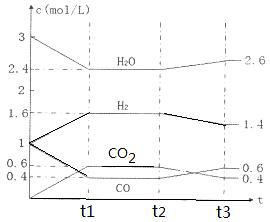
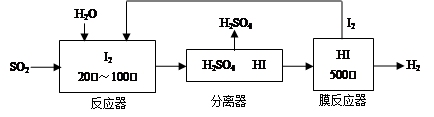
 CO(g)��H2(g)��
CO(g)��H2(g)�� O2(g)=H2O(g)����H2����242.0 kJ��mol��1
O2(g)=H2O(g)����H2����242.0 kJ��mol��1 CO2(g)��H2O(g)
CO2(g)��H2O(g)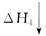

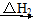 CO(g)��H2O(g)��
CO(g)��H2O(g)��