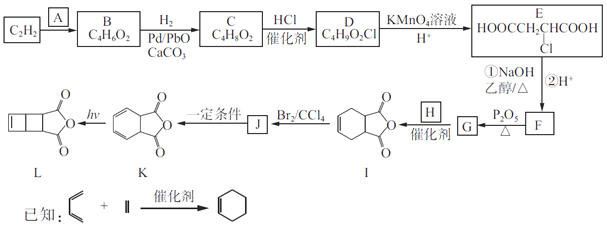
��Ŀ����
����Ŀ��Ϊ��ȥ�����е�Ca2+. Mg2+. SO42-�Լ���ɳ�����ʣ�ijͬѧ�����һ���Ʊ����ε�ʵ�鷽�����������£����ڳ������Լ��Թ�����
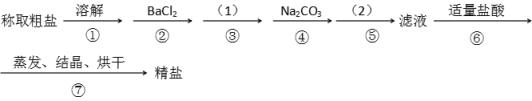
��1���ڢܲ��У�д����Ӧ�����ӷ���ʽ���������Һ��Ca2+����Ҫ������ʽΪCaCl2��_______________ ��____________��
��2��ʵ�鷽���ģ�1����Ӧʹ�ó����Լ��Ļ�ѧʽ__________�����������ӷ���ʽ��__________����ʵ�鷽���ģ�2���еIJ���������_______��
��3����ʵ����Ʒ����Ż��ĽǶȷ�������ںܿ͢ɷ�ߵ�____________����ǡ������������˵�����ɡ�___________________________________________��
��4���ж�BaCl2�ѹ����ķ�����_________________________________________________��
���𰸡�Ca2�� + CO32�� = CaCO3�� Ba2��+ CO32�� = BaCO3�� NaOH 2OH-+Mg2+=Mg(OH)2�� ���� �� ������BaCl2����Ҫ��Na2CO3��ȥ ����ȡ�ϲ���Һ�����μ�BaCl2��Һ�����������ɣ���BaCl2�����������𰸺������ɣ�
��������
��1��Ca2+��CO32-��Ӧ����CaCO3������Ba2��CO32-��Ӧ����BaCO3������
��2���������ƿ��Խ���Һ�е�þ���ӳ�����
��3��̼���Ʊ�������Ȼ����ĺ������,���ܵߵ����������ƺ��Ȼ����ļ�����˳��Ҫ��
��4���Ȼ�������ʱ,��Һ�в��Ậ�����������,���Լ����Ƿ��������������ȷ���Ȼ����Ƿ������
��1��̼���Ƶ������ǽ���Һ�еĸ����Ӻ����ı����ӳ�������,������Ӧ�����ӷ���ʽ�ֱ�Ϊ��Ca2�� + CO32�� = CaCO3����Ba2��+ CO32�� = BaCO3����
�������Ca2�� + CO32�� = CaCO3����Ba2��+ CO32�� = BaCO3����
��2���������ƿ�����Һ�е�þ�����γ�Mg(OH)2������Ȼ������Գ�ȥMg2+�����ӷ���ʽΪ��2OH-+Mg2+=Mg(OH)2����
�����Ϊ��NaOH��2OH-+Mg2+=Mg(OH)2�������ˡ�
��3��̼���Ʊ�������Ȼ����ĺ������,����̼���Ƽȿ��Խ��������Ӹ����ӳ�ȥ,�ֿ��Խ������ı����ӳ�ȥ,����Ba2+��������Ӱ�쾫�εĴ��ȣ��ʲ��ܵߵ��������Ϊ��������BaCl2����Ҫ��Na2CO3��ȥ��
��4���Ȼ�������ʱ����Һ�в��Ậ����������ӣ����Լ�����Һ���Ƿ��������������ȷ���Ȼ����Ƿ����������������:ȡ���ú���ϲ���Һ���ڵ�ΰ���(��ȡ�����ϲ���Һ���Թ���)���ٵ���1~2��BaCl2��Һ,����Һδ�����,�����BaCl2�ѹ�����������ȷ����: ����ȡ�ϲ���Һ�����μ�BaCl2��Һ�����������ɣ���BaCl2������